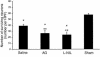Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice - PubMed (original) (raw)
. 1999 Sep;104(5):647-56.
doi: 10.1172/JCI6670.
P M Kochanek, C E Dixon, R S Clark, J A Carcillo, J K Schiding, M Chen, S R Wisniewski, T M Carlos, D Williams, S T DeKosky, S C Watkins, D W Marion, T R Billiar
Affiliations
- PMID: 10487779
- PMCID: PMC408535
- DOI: 10.1172/JCI6670
Inducible nitric oxide synthase is an endogenous neuroprotectant after traumatic brain injury in rats and mice
E H Sinz et al. J Clin Invest. 1999 Sep.
Abstract
Nitric oxide (NO) derived from the inducible isoform of NO synthase (iNOS) is an inflammatory product implicated both in secondary damage and in recovery from brain injury. To address the role of iNOS in experimental traumatic brain injury (TBI), we used 2 paradigms in 2 species. In a model of controlled cortical impact (CCI) with secondary hypoxemia, rats were treated with vehicle or with 1 of 2 iNOS inhibitors (aminoguanidine and L-N-iminoethyl-lysine), administered by Alzet pump for 5 days and 1. 5 days after injury, respectively. In a model of CCI, knockout mice lacking the iNOS gene (iNOS(-/-)) were compared with wild-type (iNOS(+/+)) mice. Functional outcome (motor and cognitive) during the first 20 days after injury, and histopathology at 21 days, were assessed in both studies. Treatment of rats with either of the iNOS inhibitors after TBI significantly exacerbated deficits in cognitive performance, as assessed by Morris water maze (MWM) and increased neuron loss in vulnerable regions (CA3 and CA1) of hippocampus. Uninjured iNOS(+/+) and iNOS(-/-) mice performed equally well in both motor and cognitive tasks. However, after TBI, iNOS(-/-) mice showed markedly worse performance in the MWM task than iNOS(+/+) mice. A beneficial role for iNOS in TBI is supported.
Figures
Figure 1
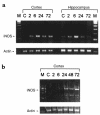
iNOS mRNA expression in rats and mice after TBI. iNOS mRNA was detected using RT-PCR. (a) In rats, iNOS mRNA was increased in injured cortex at 2, 6, 24, and 72 hours, and in ipsilateral hippocampus at 2 and 6 hours after TBI plus secondary hypoxemic insult, compared with control (n = 2 animals per group). A 138-bp PCR product is seen. (b) In C57BL/6J mice, iNOS mRNA was increased in injured cortex at 24, 48, and 72 hours after TBI, compared with control (n = 3 animals per group). A 429-bp PCR product is seen. RT-PCR for actin confirmed equal loading of RNA. M, marker; C, uninjured control.
Figure 2
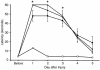
Mean latencies (± SEM) of rats to balance on a beam before and after TBI with secondary hypoxemic insult. Before injury, rats were tested for their ability to balance for up to 60 seconds on a beam. Beginning 1 day after injury, beam-balance latencies were measured daily for 5 consecutive days. All injured groups had shorter latencies (indicating impairment) on day 1 after injury. Shown are saline (filled diamonds), AG (filled circles), and L-NIL (open triangles) treatments. *P < 0.05 vs. sham (open squares; shams were not subjected to CCI or hypoxemia). There were no differences between groups treated with iNOS inhibitors vs. saline after TBI.
Figure 3
Mean latencies (± SEM) of rats to traverse a beam before and after TBI with secondary hypoxemic insult. Before injury, rats were trained to traverse the beam within 5 seconds (Before). Beginning 1 day after injury, latencies were measured daily for 5 days. All injured groups had longer (impaired) latencies on days 1–3 after injury. Shown are saline (filled diamonds), AG (filled circles), and L-NIL (open triangles) treatments. *P < 0.05 vs. sham (open squares; shams were not subjected to CCI or hypoxemia). There were no differences between groups treated with iNOS inhibitors vs. saline after TBI.
Figure 4
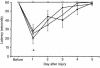
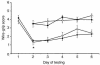
The effects of TBI with secondary hypoxemic insult and treatment with iNOS inhibitors on MWM performance in rats. Mean latencies (± SEM) to find a submerged (hidden) platform on days 14–18 after TBI. All injured groups had the longest (most impaired) latencies on day 14 after injury. However, rats treated with either of the iNOS inhibitors (AG or L-NIL) exhibited persistently higher latencies to find the platform on days 16, 17, and 18 after injury, compared with sham (open squares). Shown are saline (filled diamonds), AG (filled circles), and L-NIL (open triangles) treatments. *P < 0.05 vs. sham. Improved performance on visible-platform testing (compared with hidden-platform testing) done on days 19–20 indicates that the deficits seen were not caused by visual impairment.
Figure 5
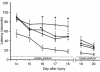
Number of surviving CA3 hippocampal neurons per HPF (×400) ± SEM at 21 days after TBI with secondary hypoxemic insult in rats treated with saline, AG, or L-NIL, and in shams. The number of surviving CA3 hippocampal neurons was reduced by TBI (*P < 0.05 for saline vs. sham). Treatment with either of the iNOS inhibitors further reduced CA3 hippocampal neuron survival (AG and L-NIL both P < 0.05 vs. *sham or **saline).
Figure 6
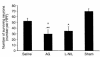
Number of surviving CA1 hippocampal neurons per HPF (×400) ± SEM at 21 days after TBI with secondary hypoxemic insult in rats treated with saline, AG, or L-NIL, and in shams. Treatment with either of the iNOS inhibitors reduced CA1 hippocampal neuron survival (*AG and L-NIL both P < 0.05 vs. sham). Rats treated with AG exhibited reduced CA1 neuron survival vs. saline-treated rats after TBI (**P < 0.05).
Figure 7
Assessment of motor function in mice using wire-grip scores (mean ± SEM). Uninjured iNOS+/+ (open squares) and iNOS–/– (filled squares) mice performed equally well in this task, as assessed for 5 days. In 2 separate groups of mice, both iNOS+/+ (open circles) and iNOS–/– (filled circles) exhibited reduced scores (deficits) in this task after injury (day 1 shows preinjury score; day 2 of testing represents the first postinjury day). *P < 0.05 vs. preinjury scores. There were no differences between iNOS+/+ and iNOS–/– groups after injury.
Figure 8
Spatial memory performance using an MWM paradigm in mice. Mean latencies (± SEM) to find a submerged (hidden) platform on days 14–18 after TBI. Uninjured iNOS+/+ (open squares) and iNOS–/– (filled squares) mice performed equally well on this task, as assessed for 5 days. After injury, both iNOS+/+ (open circles) and iNOS–/– (filled circles) mice showed impaired performance, as assessed for 5 days (vs. either uninjured group). After TBI, iNOS–/– mice showed markedly impaired performance (*P < 0.05 vs. injured iNOS+/+ mice on days 15 and 18). Improved performance on visible-platform testing (days 19–20) indicates that the deficits seen were not due to visual impairment.
Similar articles
- Inducible nitric oxide synthase expression after traumatic brain injury and neuroprotection with aminoguanidine treatment in rats.
Wada K, Chatzipanteli K, Kraydieh S, Busto R, Dietrich WD. Wada K, et al. Neurosurgery. 1998 Dec;43(6):1427-36. doi: 10.1097/00006123-199812000-00096. Neurosurgery. 1998. PMID: 9848857 - Selective inhibition of inducible nitric oxide synthase reduces neurological deficit but not cerebral edema following traumatic brain injury.
Louin G, Marchand-Verrecchia C, Palmier B, Plotkine M, Jafarian-Tehrani M. Louin G, et al. Neuropharmacology. 2006 Feb;50(2):182-90. doi: 10.1016/j.neuropharm.2005.08.020. Epub 2005 Oct 19. Neuropharmacology. 2006. PMID: 16242164 - 1400W, a potent selective inducible NOS inhibitor, improves histopathological outcome following traumatic brain injury in rats.
Jafarian-Tehrani M, Louin G, Royo NC, Besson VC, Bohme GA, Plotkine M, Marchand-Verrecchia C. Jafarian-Tehrani M, et al. Nitric Oxide. 2005 Mar;12(2):61-9. doi: 10.1016/j.niox.2004.12.001. Epub 2005 Jan 21. Nitric Oxide. 2005. PMID: 15740979 - Inducible nitric oxide synthase: a little bit of good in all of us.
Kubes P. Kubes P. Gut. 2000 Jul;47(1):6-9. doi: 10.1136/gut.47.1.6. Gut. 2000. PMID: 10861252 Free PMC article. Review. - Inducible nitric oxide synthase in the central nervous system.
Sparrow JR. Sparrow JR. J Mol Neurosci. 1994-1995;5(4):219-29. doi: 10.1007/BF02736723. J Mol Neurosci. 1994. PMID: 7577365 Review.
Cited by
- Inhalational Gases for Neuroprotection in Traumatic Brain Injury.
Shin SS, Hwang M, Diaz-Arrastia R, Kilbaugh TJ. Shin SS, et al. J Neurotrauma. 2021 Oct 1;38(19):2634-2651. doi: 10.1089/neu.2021.0053. Epub 2021 Jun 8. J Neurotrauma. 2021. PMID: 33940933 Free PMC article. - Adrenomedullin as a growth and cell fate regulatory factor for adult neural stem cells.
Martínez-Herrero S, Larráyoz IM, Ochoa-Callejero L, García-Sanmartín J, Martínez A. Martínez-Herrero S, et al. Stem Cells Int. 2012;2012:804717. doi: 10.1155/2012/804717. Epub 2012 Sep 24. Stem Cells Int. 2012. PMID: 23049570 Free PMC article. - Nitric oxide signalling in the brain and its control of bodily functions.
Chachlaki K, Prevot V. Chachlaki K, et al. Br J Pharmacol. 2020 Dec;177(24):5437-5458. doi: 10.1111/bph.14800. Epub 2019 Sep 8. Br J Pharmacol. 2020. PMID: 31347144 Free PMC article. Review. - Traumatic brain injury disrupts cerebrovascular tone through endothelial inducible nitric oxide synthase expression and nitric oxide gain of function.
Villalba N, Sonkusare SK, Longden TA, Tran TL, Sackheim AM, Nelson MT, Wellman GC, Freeman K. Villalba N, et al. J Am Heart Assoc. 2014 Dec;3(6):e001474. doi: 10.1161/JAHA.114.001474. J Am Heart Assoc. 2014. PMID: 25527626 Free PMC article. - Neurodegeneration and glia response in rat hippocampus following nitro-L-arginine methyl ester (L-NAME).
Harry GJ, Sills R, Schlosser MJ, Maier WE. Harry GJ, et al. Neurotox Res. 2001 Jul;3(3):307-19. doi: 10.1007/BF03033270. Neurotox Res. 2001. PMID: 15111256
References
- Lassmann H. Basic mechanisms of brain inflammation. J Neural Transm Suppl. 1997;50:183–190. - PubMed
- McGeer PL, McGeer EG. The inflammatory response system of brain: implications for therapy of Alzheimer and other neurodegenerative diseases. Brain Res Brain Res Rev. 1995;21:195–218. - PubMed
- Pfister HW, Scheld W. Brain injury in bacterial meningitis: therapeutic implications. Curr Opin Neurol. 1997;10:254–259. - PubMed
- Feuerstein GZ, Wang X, Barone FC. Inflammatory gene expression in cerebral ischemia and trauma. Potential new therapeutic targets. Ann NY Acad Sci. 1997;825:179–193. - PubMed
- Hallenbeck JM. Significance of the inflammatory response in brain ischemia. Acta Neurochir Suppl (Wien) 1996;66:27–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS38620/NS/NINDS NIH HHS/United States
- R01 NS038620/NS/NINDS NIH HHS/United States
- P01 NS030318/NS/NINDS NIH HHS/United States
- GM-44100/GM/NIGMS NIH HHS/United States
- NS-30318/NS/NINDS NIH HHS/United States
- P50 NS030318/NS/NINDS NIH HHS/United States
- R01 GM044100/GM/NIGMS NIH HHS/United States
- R37 GM044100/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous