Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation - PubMed (original) (raw)
Nuclear accumulation of mutated beta-catenin in hepatocellular carcinoma is associated with increased cell proliferation
J T Nhieu et al. Am J Pathol. 1999 Sep.
Abstract
Inappropriate activation of the Wnt pathway resulting from beta-catenin gene alterations has recently been implicated in the development of hepatocellular carcinoma (HCC). To explore the in vivo effects of mutated beta-catenin, HCC specimens from 32 patients carrying one or several tumors were screened for somatic mutations in exon 3 of the beta-catenin gene, and the expression and subcellular localization of beta-catenin was studied by immunohistochemistry. Missense mutations or interstitial deletions in beta-catenin exon 3 were detected in 12 of 35 (34%) HCC samples. After immunostaining, most tumors exhibited increased membranous and/or cytoplasmic expression of beta-catenin compared with adjacent nontumoral liver. Strong nuclear accumulation of beta-catenin was observed either focally or uniformly in 15 of 35 (43%) tumor specimens, but not in cirrhotic nodules or dysplastic liver cells in adjacent liver. Aberrant nuclear expression of beta-catenin was significantly associated with the presence of mutations in the beta-catenin gene (P < 0.005). Moreover, nuclear beta-catenin staining correlated significantly with increased Ki-67 proliferative index in tumor (P < 0.001) and seemed to be associated with poor outcome in patients with HCC. In conclusion, our data indicate that activation of the Wnt/beta-catenin pathway in HCC results mainly from somatic mutations in the beta-catenin gene and may promote tumor progression by stimulating tumor cell proliferation.
Figures
Figure 1.
Oncogenic mutations of one β-catenin allele in HCC. A: Genomic PCR of β-catenin intron 2 to exon 4 sequences showing the normal PCR product (1.1 kbp) in tumors carrying wild-type (lanes 1 and 3) or point mutated β-catenin (lane 4, Patient 1) and a smaller product (0.5 kbp) corresponding to the deleted allele in Patient 12 (lane 2). B: Sequence analysis showing three point mutations in tumor from Patient 7. C: Sequence analysis of the tumorous (T) and nontumorous (NT) liver from Patient 11 showing an interstitial deletion spanning 553 bp of exon 3, intron 3, and exon 4. The dinucleotide CA was present at both ends of the deletion. D: Summary of amino acid substitutions found in 10 different tumors.
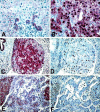
Figure 2.
A, B, C, and E: β-catenin immunolocalization. A: In nontumoral liver, a thin membranous signal delineates the hepatocytes, and bile ductules show strong membranous and pale cytoplasmic staining. B: Overexpression of β-catenin in the cytoplasm and in most nuclei in a HCC case harboring a mutation in β-catenin exon 3. C: Tumoral invasion of a portal vein with strong cytoplasmic and nuclear β-catenin staining. E: Heterogeneous tumor showing strong cytoplasmic and nuclear β-catenin staining in most cells in some areas (left and right sides), and milder staining with only few positive nuclei in another area (center). D and F: MIB-1 (Ki-67) immunostaining. D: A serial section of the portal tumoral invasion shown in C, showing a high proliferative index. F: A serial section of the heterogeneous tumor in E, showing colocalization of MIB-1 and β-catenin stainings. Original magnifications, ×250 (A, B) and ×100 (C-F).
Similar articles
- beta-Catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance.
Wong CM, Fan ST, Ng IO. Wong CM, et al. Cancer. 2001 Jul 1;92(1):136-45. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. Cancer. 2001. PMID: 11443619 - Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival.
Inagawa S, Itabashi M, Adachi S, Kawamoto T, Hori M, Shimazaki J, Yoshimi F, Fukao K. Inagawa S, et al. Clin Cancer Res. 2002 Feb;8(2):450-6. Clin Cancer Res. 2002. PMID: 11839663 - Tumor heterogeneity in small hepatocellular carcinoma: analysis of tumor cell proliferation, expression and mutation of p53 AND beta-catenin.
An FQ, Matsuda M, Fujii H, Tang RF, Amemiya H, Dai YM, Matsumoto Y. An FQ, et al. Int J Cancer. 2001 Aug 15;93(4):468-74. doi: 10.1002/ijc.1367. Int J Cancer. 2001. PMID: 11477549 - Overview of the molecular biology of hepatocellular neoplasms and hepatoblastomas of the mouse liver.
Kim Y, Sills RC, Houle CD. Kim Y, et al. Toxicol Pathol. 2005;33(1):175-80. doi: 10.1080/01926230590522130. Toxicol Pathol. 2005. PMID: 15805069 Review. - Truncated form of beta-catenin and reduced expression of wild-type catenins feature HepG2 human liver cancer cells.
Carruba G, Cervello M, Miceli MD, Farruggio R, Notarbartolo M, Virruso L, Giannitrapani L, Gambino R, Montalto G, Castagnetta L. Carruba G, et al. Ann N Y Acad Sci. 1999;886:212-6. doi: 10.1111/j.1749-6632.1999.tb09419.x. Ann N Y Acad Sci. 1999. PMID: 10667222 Review. No abstract available.
Cited by
- Selective Retention of an Inactive Allele of the DKK2 Tumor Suppressor Gene in Hepatocellular Carcinoma.
Lin YF, Li LH, Lin CH, Tsou MH, Chuang MT, Wu KM, Liao TL, Li JC, Wang WJ, Tomita A, Tomita B, Huang SF, Tsai SF. Lin YF, et al. PLoS Genet. 2016 May 20;12(5):e1006051. doi: 10.1371/journal.pgen.1006051. eCollection 2016 May. PLoS Genet. 2016. PMID: 27203079 Free PMC article. - Hepatocellular carcinoma development induced by conditional beta-catenin activation in Lkb1+/- mice.
Miyoshi H, Deguchi A, Nakau M, Kojima Y, Mori A, Oshima M, Aoki M, Taketo MM. Miyoshi H, et al. Cancer Sci. 2009 Nov;100(11):2046-53. doi: 10.1111/j.1349-7006.2009.01284.x. Epub 2009 Jul 9. Cancer Sci. 2009. PMID: 19671058 Free PMC article. - Generation and analysis of genetically defined liver carcinomas derived from bipotential liver progenitors.
Zender L, Xue W, Cordón-Cardo C, Hannon GJ, Lucito R, Powers S, Flemming P, Spector MS, Lowe SW. Zender L, et al. Cold Spring Harb Symp Quant Biol. 2005;70:251-61. doi: 10.1101/sqb.2005.70.059. Cold Spring Harb Symp Quant Biol. 2005. PMID: 16869761 Free PMC article. - Dickkopfs and Wnt/β-catenin signalling in liver cancer.
Fatima S, Lee NP, Luk JM. Fatima S, et al. World J Clin Oncol. 2011 Aug 10;2(8):311-25. doi: 10.5306/wjco.v2.i8.311. World J Clin Oncol. 2011. PMID: 21876852 Free PMC article. - Caspase-mediated cleavage of beta-catenin precedes drug-induced apoptosis in resistant cancer cells.
Senthivinayagam S, Mishra P, Paramasivam SK, Yallapragada S, Chatterjee M, Wong L, Rana A, Rana B. Senthivinayagam S, et al. J Biol Chem. 2009 May 15;284(20):13577-13588. doi: 10.1074/jbc.M900248200. Epub 2009 Mar 16. J Biol Chem. 2009. PMID: 19289465 Free PMC article.
References
- Murray CJL, Lopez AD: Mortality by cause for eight regions of the world: global burden of disease study. Lancet 1997, 349:1269-1276 - PubMed
- Nagai H, Buendia MA: Oncogenes, tumor suppressors, and co-factors in hepatocellular carcinoma. Koshy R Caselman W eds. Hepatitis B Virus. 1998, :pp 182-218 Imperial College Press, London
- Nagai H, Pineau P, Tiollais P, Buendia MA, Dejean A: Comprehensive allelotyping of human hepatocellular carcinoma. Oncogene 1997, 14:2927-2933 - PubMed
- Murakami Y, Hayashi K, Hirohashi S, Sekiya T: Aberrations of the tumor suppressor p53 and retinoblastoma genes in human hepatocellular carcinomas. Cancer Res 1991, 51:5520-5525 - PubMed
- Hsu HC, Peng SY, Lai PL, Sheu JC, Chen DS, Lin LI, Slagle BL, Butel JS: Allelotype and loss of heterozygosity of p53 in primary and recurrent hepatocellular carcinomas. Cancer 1994, 73:42-47 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous