Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry - PubMed (original) (raw)
Vascular channel formation by human melanoma cells in vivo and in vitro: vasculogenic mimicry
A J Maniotis et al. Am J Pathol. 1999 Sep.
Abstract
Tissue sections from aggressive human intraocular (uveal) and metastatic cutaneous melanomas generally lack evidence of significant necrosis and contain patterned networks of interconnected loops of extracellular matrix. The matrix that forms these loops or networks may be solid or hollow. Red blood cells have been detected within the hollow channel components of this patterned matrix histologically, and these vascular channel networks have been detected in human tumors angiographically. Endothelial cells were not identified within these matrix-embedded channels by light microscopy, by transmission electron microscopy, or by using an immunohistochemical panel of endothelial cell markers (Factor VIII-related antigen, Ulex, CD31, CD34, and KDR[Flk-1]). Highly invasive primary and metastatic human melanoma cells formed patterned solid and hollow matrix channels (seen in tissue sections of aggressive primary and metastatic human melanomas) in three-dimensional cultures containing Matrigel or dilute Type I collagen, without endothelial cells or fibroblasts. These tumor cell-generated patterned channels conducted dye, highlighting looping patterns visualized angiographically in human tumors. Neither normal melanocytes nor poorly invasive melanoma cells generated these patterned channels in vitro under identical culture conditions, even after the addition of conditioned medium from metastatic pattern-forming melanoma cells, soluble growth factors, or regimes of hypoxia. Highly invasive and metastatic human melanoma cells, but not poorly invasive melanoma cells, contracted and remodeled floating hydrated gels, providing a biomechanical explanation for the generation of microvessels in vitro. cDNA microarray analysis of highly invasive versus poorly invasive melanoma tumor cells confirmed a genetic reversion to a pluripotent embryonic-like genotype in the highly aggressive melanoma cells. These observations strongly suggest that aggressive melanoma cells may generate vascular channels that facilitate tumor perfusion independent of tumor angiogenesis.
Figures
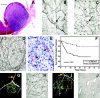
Figure 1.
Histology of the melanoma microcirculation, its prognostic significance, and in vivo functional correlations. A: Primary uveal melanoma in the choroid of a patient who died of metastatic melanoma 3 years after the eye was removed. Note the absence of necrosis. Hematoxylin-eosin stain; scale bar, 2 mm. B: Light microscopic view of PAS-stained tissue sections of primary human uveal melanoma (arrow demonstrates a sinusoid with red blood cells). C: Uveal melanoma metastatic to the liver, showing patterns of interconnected loops forming networks anastomosing to sinusoids (arrow) containing red blood cells. D: PAS-stained histological section of metastatic cutaneous melanoma containing multiple networks. E: Hematoxylin-eosin stained section of primary uveal melanoma; the structures corresponding to the PAS-positive components of loops and networks consist of solid cords (arrows) that connect to round channels or tubes containing red blood cells (arrowheads). Note that these tubules are lined externally by melanoma cells and no endothelial cells are identified. F: Kaplan-Meier survival curves for deaths from metastatic uveal melanoma: melanomas without networks versus melanomas with networks (after Folberg, et al 4 ). G: Angiogram of uveal melanoma taken after intravenous injection of indocyanine green and photographed with a laser scanning confocal ophthalmic recording device. The vessels forming an inverted “Y,” highlighted by the yellow arrow, are within the retina. The anastomosing loops between the two inverted branches of the “Y” formed by the retinal vessels and enclosed by the yellow box are deeper within the tumor and represent the microcirculation of this melanoma. This eye was subsequently removed and studied histologically (shown in H). H: Tissue section stained by PAS, without hematoxylin counterstain, taken from the tumor at the location enclosed by the box from the angiogram (G). Many PAS-positive loops forming networks are seen in this tissue, corresponding precisely to the loops seen in the angiogram. I: Angiogram of uveal melanoma taken after intravenous injection of indocyanine green and photographed with a laser scanning confocal ophthalmic recording device. In contrast to the angiogram shown in G, looping vessels are not seen within the tumor (yellow box). The vessels within the tumor are parallel straight vessels. This eye was subsequently removed and studied histologically (shown in J). J: Tissue section stained by PAS without hematoxylin taken from the area in the tumor enclosed by the box on the angiogram (I). Dilated normal choroidal vessels are identified and correspond to the straight parallel vessels on the angiogram. Scale bar, 2 mm (A). Original magnifications, ×40 (B, C, H, J), ×80 (D), ×120 E. Tissue stains: hematoxylin-eosin (A and E), PAS without hematoxylin counterstaining, photographed with a green filter (B-D, H-J).
Figure 2.
Transmission electron microscopy of melanoma microcirculation patterns. A: Tissue section of primary uveal melanoma stained by PAS without hematoxylin counterstain (same tumor as illustrated in Figure 1E ▶ ). Areas from this tumor were microdissected and studied by transmission electron microscopy. B: Scanning magnification transmission electron micrograph of a tubule containing a single-file column of red blood cells. This vascular channel is lined by a thin basal lamina (arrowheads) corresponding to the walls of the channel seen by conventional light microscopy. There are no endothelial cells lining the tubule. Tumor cells containing melanosomes and premelanosomes lie external to the basal lamina. C: Higher magnification of Figure 2C ▶ illustrating premelanosomes and melanosomes within the tumor. D: Hematoxylin-eosin-stained tissue section of primary uveal melanoma. A vascular channel containing red blood cells is lined externally by tumor cells; endothelial cells are not identified. Original magnifications, ×40 (A), ×10,000 (B), ×30,000 (C), ×100 (D).
Figure 3.
Immunohistochemistry of melanoma microcirculation patterns. A: Proliferative diabetic retinopathy, a classic example of an angiogenic response, was used as one control. The discrete vessels stained for Factor VIII-related antigen are not interconnected and do not form loops that encircle domains of tissue in histological section (as shown in Figure 1, A-E and H ▶ ). B: Higher magnification of proliferative diabetic retinopathy stained for Factor VIII-related antigen. At least five endothelial cell nuclei are identified lining this small vessel. Cells containing brown pigment represent iron in macrophages and reflects vascular incompetence in this neo-angiogenic response. C: Tissue section taken from primary uveal melanoma, stained by PAS without hematoxylin. This patient died of metastatic melanoma. Note the interconnected loops. D: Same tumor as illustrated in C, stained for Factor VIII-related antigen (appears red). The loops stain only focally and discontinuously. E: The same tumor as illustrated in C and D, stained for CD31. Staining of this pattern with CD31 is even less evident than with Factor VIII-related antigen. F: Primary uveal melanoma showing intense CD31 staining (appears red) of tumor cells adjacent to the lumen (*) of intratumoral vascular tube lacking endothelial lining. This patient died of metastasis 6 years after the eye was removed. G: Higher magnification of tumor depicted in D, also stained for Factor VIII-related antigen. Only a segment of this otherwise hollow channel (*) stains for Factor VIII-related antigen. The remainder of the tube is vacant (*). Endothelial cell nuclei are not identified. H: Tissue section stained by Ulex europaeus agglutinin I (appears red) and counterstained with hematoxylin. Endothelial cell nuclei are not identified. Ulex labels the contents of the lumen around the red blood cells. Tumor cells are identified external to the vessel. I: Tissue section stained with CD34 and counterstained with hematoxylin; endothelial cell nuclei are not identified. CD34 labels the lumen contents between the red blood cells. Tumor cells are situated external to the vessel. J: Tissue section stained for KDR (appears red) and counterstained with hematoxylin; endothelial cell nuclei are not identified. The lumen contents are labeled with KDR. Tumor cells are situated external to the vascular channel. K: Tissue section double labeled for CD31 (red chromogen with direct illumination) and L, S-100 protein (same field viewed by reflectance microscopy; immunogold). The vascular channels do not stain for CD31 but cells apposed externally to the vascular channel lumen are positive for S-100 protein, a reaction consistent with melanoma, but not endothelial cells. Tissue stains: A, B, D,F, Factor VIII-related antigen, counterstained with hematoxylin; C, PAS without hematoxylin counterstain; E and G, CD31 counterstained with hematoxylin; H, Ulex europaeus agglutinin I counterstained with hematoxylin; I, CD34, counterstained with hematoxylin; J, KDR, counterstained with hematoxylin; K, CD31 (red chromogen) photographed by direct illumination; L, S-100 protein conjugated to immunogold (same section, same field as K) photographed with epipolarization microscopy. Original magnifications, ×20 (A, C, D, E, K, and L). Scale bar, 25 μm for B,F,G, and H.
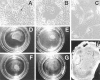
Figure 4.
A: PAS-stained phase contrast micrograph of 3D culture of highly invasive M619 human uveal melanoma cells forming patterned networks after 1 week on Matrigel. B: Metastatic uveal melanoma MUM-2B forming patterned networks on dilute Type I collagen (stained with PAS to highlight basement membranes). C: Metastatic cutaneous melanoma C8161 also formed patterned networks after 1 week on Matrigel. By contrast, poorly invasive OCM-1A cells do not form patterned networks on Matrigel (D) or on dilute Type I collagen gels (E). F: Histological cross-section of a 2-week, 3D culture of cell line M619 showing both large sinusoidal structures and smaller tubular structures embedded in the monolayer (arrow). G: Fluorescence localization of Texas red dye at the moment of microinjection into a large sinusoid of a mature vessel-forming 3D culture of pure MUM-2B cells. H: The dye distributes through smaller tumor-generated vessels in 30 minutes. Compare with Figure 1G ▶ , the confocal angiogram taken of a patient with a uveal melanoma. Original magnifications, ×200 (F); ×40 (all others).
Figure 5.
Physical and deregulated characteristics of uveal melanoma vessels. A: Micromanipulation of a tumor-generated vessel formed by M619 melanoma cells and removal of cells (small black arrow) from the acellular vascular channel, and all others. B: subsequent deformation of the same tubular structure for measurement of its mechanical response to applied strain. C: Cord formation by cultured human endothelial cells under identical media and culture conditions shown in A and B. Note that all of the endothelial cells are recruited into tessellations, unlike spheroidal nests of aggressive cultured melanoma cells which are encircled by acellular microvessels of varying diameters (A and B). D-G: Comparison of collagen gel contraction after seeding with HUVECs or tumor cells. Endothelial cells (D) contracted the floating gels in 48 hours, as do aggressive primary C918 intraocular melanoma cells (E) and metastatic C8161 cutaneous melanoma cells (F); however, poorly invasive OCM-1A primary melanoma cells (G) did not contract the floating gels even after 3 weeks’ observation. H: Concomitant gel contraction and tube formation on floating hydrated gel. Highly invasive M619 melanoma cells contract the gel and form networks as demonstrated by labeling of tumor cell mitochondria with rhodamine 123.
Comment in
- Tumor plasticity allows vasculogenic mimicry, a novel form of angiogenic switch. A rose by any other name?
Bissell MJ. Bissell MJ. Am J Pathol. 1999 Sep;155(3):675-9. doi: 10.1016/S0002-9440(10)65164-4. Am J Pathol. 1999. PMID: 10487823 Free PMC article. Review. No abstract available. - Vasculogenic mimicry in tumors. Fact or artifact?
Fausto N. Fausto N. Am J Pathol. 2000 Feb;156(2):359. doi: 10.1016/S0002-9440(10)64738-4. Am J Pathol. 2000. PMID: 10666363 Free PMC article. No abstract available. - Additional literature on "vasculogenic mimicry" not cited.
Shubik P, Warren BA. Shubik P, et al. Am J Pathol. 2000 Feb;156(2):736. doi: 10.1016/S0002-9440(10)64778-5. Am J Pathol. 2000. PMID: 10667912 Free PMC article. No abstract available.
Similar articles
- Vasculogenic mimicry and tumor angiogenesis.
Folberg R, Hendrix MJ, Maniotis AJ. Folberg R, et al. Am J Pathol. 2000 Feb;156(2):361-81. doi: 10.1016/S0002-9440(10)64739-6. Am J Pathol. 2000. PMID: 10666364 Free PMC article. Review. - Presence of a fluid-conducting meshwork in xenografted cutaneous and primary human uveal melanoma.
Clarijs R, Otte-Höller I, Ruiter DJ, de Waal RM. Clarijs R, et al. Invest Ophthalmol Vis Sci. 2002 Apr;43(4):912-8. Invest Ophthalmol Vis Sci. 2002. PMID: 11923228 - Cooperative interactions of laminin 5 gamma2 chain, matrix metalloproteinase-2, and membrane type-1-matrix/metalloproteinase are required for mimicry of embryonic vasculogenesis by aggressive melanoma.
Seftor RE, Seftor EA, Koshikawa N, Meltzer PS, Gardner LM, Bilban M, Stetler-Stevenson WG, Quaranta V, Hendrix MJ. Seftor RE, et al. Cancer Res. 2001 Sep 1;61(17):6322-7. Cancer Res. 2001. PMID: 11522618 - Tumor cell plasticity in uveal melanoma: microenvironment directed dampening of the invasive and metastatic genotype and phenotype accompanies the generation of vasculogenic mimicry patterns.
Folberg R, Arbieva Z, Moses J, Hayee A, Sandal T, Kadkol S, Lin AY, Valyi-Nagy K, Setty S, Leach L, Chévez-Barrios P, Larsen P, Majumdar D, Pe'er J, Maniotis AJ. Folberg R, et al. Am J Pathol. 2006 Oct;169(4):1376-89. doi: 10.2353/ajpath.2006.060223. Am J Pathol. 2006. PMID: 17003493 Free PMC article. - Vasculogenic mimicry.
Folberg R, Maniotis AJ. Folberg R, et al. APMIS. 2004 Jul-Aug;112(7-8):508-25. doi: 10.1111/j.1600-0463.2004.apm11207-0810.x. APMIS. 2004. PMID: 15563313 Review.
Cited by
- Tumor angiogenesis and lymphangiogenesis: tumor/endothelial crosstalk and cellular/microenvironmental signaling mechanisms.
Gomes FG, Nedel F, Alves AM, Nör JE, Tarquinio SB. Gomes FG, et al. Life Sci. 2013 Feb 7;92(2):101-7. doi: 10.1016/j.lfs.2012.10.008. Epub 2012 Nov 21. Life Sci. 2013. PMID: 23178150 Free PMC article. Review. - Endothelial Progenitors Exist within the Kidney and Lung Mesenchyme.
Sims-Lucas S, Schaefer C, Bushnell D, Ho J, Logar A, Prochownik E, Gittes G, Bates CM. Sims-Lucas S, et al. PLoS One. 2013 Jun 18;8(6):e65993. doi: 10.1371/journal.pone.0065993. Print 2013. PLoS One. 2013. PMID: 23823180 Free PMC article. - Hypoxia-independent drivers of melanoma angiogenesis.
Meierjohann S. Meierjohann S. Front Oncol. 2015 May 5;5:102. doi: 10.3389/fonc.2015.00102. eCollection 2015. Front Oncol. 2015. PMID: 26000250 Free PMC article. Review. - Molecular Mechanism and Approach in Progression of Meningioma.
Shao Z, Liu L, Zheng Y, Tu S, Pan Y, Yan S, Wei Q, Shao A, Zhang J. Shao Z, et al. Front Oncol. 2020 Sep 11;10:538845. doi: 10.3389/fonc.2020.538845. eCollection 2020. Front Oncol. 2020. PMID: 33042832 Free PMC article. Review. - Multiple myeloma macrophages: pivotal players in the tumor microenvironment.
Berardi S, Ria R, Reale A, De Luisi A, Catacchio I, Moschetta M, Vacca A. Berardi S, et al. J Oncol. 2013;2013:183602. doi: 10.1155/2013/183602. Epub 2013 Jan 30. J Oncol. 2013. PMID: 23431298 Free PMC article.
References
- Folkman J: Clinical applications of research on angiogenesis. Seminars in Medicine of the Beth Israel Hospital, Boston. New Engl J Med 1995, 333:1757-1763 - PubMed
- Risau W: Mechanisms of angiogenesis. Nature 1997, 386:671-674 - PubMed
- Weidner N: Tumoral vascularity as a prognostic factor in cancer patients: the evidence continues to grow. J Pathol 1998, 184:119-122 - PubMed
- Folberg R, Rummelt V, Parys-Van Ginderdeuren R, Hwang T, Woolson RF, Pe’er J, Gruman LM: The prognostic value of tumor blood vessel morphology in primary uveal melanoma. Ophthalmology 1993, 100:1389-1398 - PubMed
- McLean IW, Foster WD, Zimmerman LE, Gamel JW: Modifications of Callender’s classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol 1983, 96:502-509 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY010457/EY/NEI NIH HHS/United States
- R01 CA59702/CA/NCI NIH HHS/United States
- R01 CA80318/CA/NCI NIH HHS/United States
- R01 CA059702/CA/NCI NIH HHS/United States
- R01 EY10457/EY/NEI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical