Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease - PubMed (original) (raw)
Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease
L F Lue et al. Am J Pathol. 1999 Sep.
Abstract
We have characterized amyloid beta peptide (Abeta) concentration, Abeta deposition, paired helical filament formation, cerebrovascular amyloid angiopathy, apolipoprotein E (ApoE) allotype, and synaptophysin concentration in entorhinal cortex and superior frontal gyrus of normal elderly control (ND) patients, Alzheimer's disease (AD) patients, and high pathology control (HPC) patients who meet pathological criteria for AD but show no synapse loss or overt antemortem symptoms of dementia. The measures of Abeta deposition, Abeta-immunoreactive plaques with and without cores, thioflavin histofluorescent plaques, and concentrations of insoluble Abeta, failed to distinguish HPC from AD patients and were poor correlates of synaptic change. By contrast, concentrations of soluble Abeta clearly distinguished HPC from AD patients and were a strong inverse correlate of synapse loss. Further investigation revealed that Abeta40, whether in soluble or insoluble form, was a particularly useful measure for classifying ND, HPC, and AD patients compared with Abeta42. Abeta40 is known to be elevated in cerebrovascular amyloid deposits, and Abeta40 (but not Abeta42) levels, cerebrovascular amyloid angiopathy, and ApoE4 allele frequency were all highly correlated with each other. Although paired helical filaments in the form of neurofibrillary tangles or a penumbra of neurites surrounding amyloid cores also distinguished HPC from AD patients, they were less robust predictors of synapse change compared with soluble Abeta, particularly soluble Abeta40. Previous experiments attempting to relate Abeta deposition to the neurodegeneration that underlies AD dementia may have failed because they assayed the classical, visible forms of the molecule, insoluble neuropil plaques, rather than the soluble, unseen forms of the molecule.
Figures
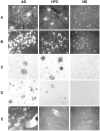
Figure 1.
Representative examples in AD patients (left panels), HPC patients (middle panels), and ND patients (right panels) of thioflavin histofluorescent plaques (A) and tangles (B), Aβ immunoreactive plaques with and without cores (C), paired helical filament occurrence around plaques (D), and cerebrovascular amyloid angiopathy (E).
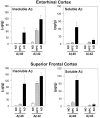
Figure 2.
Soluble and insoluble Aβ40 and Aβ42 concentrations in superior frontal gyrus and entorhinal cortex of ND, HPC, and AD patients.
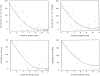
Figure 3.
Synaptic density estimates from synaptophysin Western blot analysis (synaptic density OD) as a function of entorhinal cortex and superior frontal gyrus soluble Aβ concentrations. Upper left panel: Sum of soluble Aβ40 plus Aβ42 versus synaptophysin optical density (OD) in all patients. Upper right panel: Sum of soluble Aβ40 plus Aβ42 in HPC and AD patients only. Correlations within individual structures or for soluble Aβ40 and Aβ42 alone were also highly significant and gave trends similar to those illustrated here. For example, the bottom panels show the data for soluble Aβ40 in superior frontal gyrus of all patients (left) or AD patients alone (right).
Figure 4.
Synaptic density estimates from synaptophysin Western blot analysis as a function of entorhinal cortex and superior frontal gyrus paired helical filament immunoreactive (PHF+) plaques/mm (left panel) or thioflavin histofluorescent tangles/mm (right panel). Data points for ND, HPC, and AD patients are included.
Similar articles
- Impact of sex and APOE4 on cerebral amyloid angiopathy in Alzheimer's disease.
Shinohara M, Murray ME, Frank RD, Shinohara M, DeTure M, Yamazaki Y, Tachibana M, Atagi Y, Davis MD, Liu CC, Zhao N, Painter MM, Petersen RC, Fryer JD, Crook JE, Dickson DW, Bu G, Kanekiyo T. Shinohara M, et al. Acta Neuropathol. 2016 Aug;132(2):225-234. doi: 10.1007/s00401-016-1580-y. Epub 2016 May 14. Acta Neuropathol. 2016. PMID: 27179972 Free PMC article. - Oligomeric Abeta in Alzheimer's disease: relationship to plaque and tangle pathology, APOE genotype and cerebral amyloid angiopathy.
van Helmond Z, Miners JS, Kehoe PG, Love S. van Helmond Z, et al. Brain Pathol. 2010 Mar;20(2):468-80. doi: 10.1111/j.1750-3639.2009.00321.x. Epub 2009 Jul 16. Brain Pathol. 2010. PMID: 19725829 Free PMC article. - Clusterin levels are increased in Alzheimer's disease and influence the regional distribution of Aβ.
Miners JS, Clarke P, Love S. Miners JS, et al. Brain Pathol. 2017 May;27(3):305-313. doi: 10.1111/bpa.12392. Epub 2016 Jul 8. Brain Pathol. 2017. PMID: 27248362 Free PMC article. - [Involvement of beta-amyloid in the etiology of Alzheimer's disease].
Tomiyama T. Tomiyama T. Brain Nerve. 2010 Jul;62(7):691-9. Brain Nerve. 2010. PMID: 20675873 Review. Japanese. - Is Alzheimer's disease a result of presynaptic failure? Synaptic dysfunctions induced by oligomeric beta-amyloid.
Nimmrich V, Ebert U. Nimmrich V, et al. Rev Neurosci. 2009;20(1):1-12. doi: 10.1515/revneuro.2009.20.1.1. Rev Neurosci. 2009. PMID: 19526730 Review.
Cited by
- Meta-Analysis of Serum Insulin-Like Growth Factor 1 in Alzheimer's Disease.
Ostrowski PP, Barszczyk A, Forstenpointner J, Zheng W, Feng ZP. Ostrowski PP, et al. PLoS One. 2016 May 26;11(5):e0155733. doi: 10.1371/journal.pone.0155733. eCollection 2016. PLoS One. 2016. PMID: 27227831 Free PMC article. - Neuropsychiatric Disturbances in Alzheimer's Disease: What Have We Learned from Neuropathological Studies?
Van Dam D, Vermeiren Y, Dekker AD, Naudé PJ, Deyn PP. Van Dam D, et al. Curr Alzheimer Res. 2016;13(10):1145-64. doi: 10.2174/1567205013666160502123607. Curr Alzheimer Res. 2016. PMID: 27137218 Free PMC article. Review. - Aggregation of Aß(25-35) on DOPC and DOPC/DHA bilayers: an atomic force microscopy study.
Sublimi Saponetti M, Grimaldi M, Scrima M, Albonetti C, Nori SL, Cucolo A, Bobba F, D'Ursi AM. Sublimi Saponetti M, et al. PLoS One. 2014 Dec 31;9(12):e115780. doi: 10.1371/journal.pone.0115780. eCollection 2014. PLoS One. 2014. PMID: 25551704 Free PMC article. - The Aggregation Paths and Products of Aβ42 Dimers Are Distinct from Those of the Aβ42 Monomer.
O'Malley TT, Witbold WM 3rd, Linse S, Walsh DM. O'Malley TT, et al. Biochemistry. 2016 Nov 8;55(44):6150-6161. doi: 10.1021/acs.biochem.6b00453. Epub 2016 Oct 26. Biochemistry. 2016. PMID: 27750419 Free PMC article. - New ELISAs with high specificity for soluble oligomers of amyloid β-protein detect natural Aβ oligomers in human brain but not CSF.
Yang T, Hong S, O'Malley T, Sperling RA, Walsh DM, Selkoe DJ. Yang T, et al. Alzheimers Dement. 2013 Mar;9(2):99-112. doi: 10.1016/j.jalz.2012.11.005. Epub 2013 Jan 30. Alzheimers Dement. 2013. PMID: 23375565 Free PMC article.
References
- Crystal H, Dickson D, Fuld P, Masur D, Scott R, Mehler M, Masdeu J, Kawas C, Aronson M, Wolfson L: Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology 1998, 38:1682-1687 - PubMed
- Katzman R, Terry R, DeTeresa R, Brown T, Davis P, Fuld P, Renbing X, Peck A: Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Ann Neurol 1988, 23:138-144 - PubMed
- Dickson DW, Crystal HA, Mattiace LA, Masur DM, Blau AD, Davies P, Yen SH, Aronson MK: Identification of normal and pathological aging in prospectively studied nondemented elderly humans. Neurobiol Aging 1991, 13:179-189 - PubMed
- Arrigada PA, Marzloff K, Hyman BT: Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 1992, 42:1681-1688 - PubMed
- Lue LF, Brachova L, Civin WH, Rogers J: Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J Neuropathol Exp Neurol 1996, 55:1083-1088 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous