Listeria monocytogenes exploits normal host cell processes to spread from cell to cell - PubMed (original) (raw)
Listeria monocytogenes exploits normal host cell processes to spread from cell to cell
J R Robbins et al. J Cell Biol. 1999.
Abstract
The bacterial pathogen, Listeria monocytogenes, grows in the cytoplasm of host cells and spreads intercellularly using a form of actin-based motility mediated by the bacterial protein ActA. Tightly adherent monolayers of MDCK cells that constitutively express GFP-actin were infected with L. monocytogenes, and intercellular spread of bacteria was observed by video microscopy. The probability of formation of membrane-bound protrusions containing bacteria decreased with host cell monolayer age and the establishment of extensive cell-cell contacts. After their extension into a recipient cell, intercellular membrane-bound protrusions underwent a period of bacterium-dependent fitful movement, followed by their collapse into a vacuole and rapid vacuolar lysis. Actin filaments in protrusions exhibited decreased turnover rates compared with bacterially associated cytoplasmic actin comet tails. Recovery of motility in the recipient cell required 1-2 bacterial generations. This delay may be explained by acid-dependent cleavage of ActA by the bacterial metalloprotease, Mpl. Importantly, we have observed that low levels of endocytosis of neighboring MDCK cell surface fragments occurs in the absence of bacteria, implying that intercellular spread of bacteria may exploit an endogenous process of paracytophagy.
Figures
Figure 1
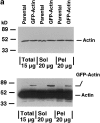
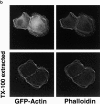
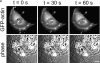
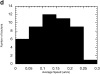
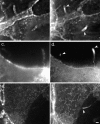
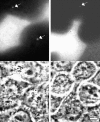
MDCK clones expressing GFP-actin. (a) Western blot stained for actin showing ratio of GFP-actin to actin in the GFP-actin MDCK clone. The upper blot was exposed for 10 s; the lower overexposed for 20 min to enable detection of GFP-actin. The ratio of actin to GFP-actin is ∼50:1. Note that GFP-actin is present in both the Triton X-100–soluble and –insoluble fractions though slightly more is soluble compared with wild-type actin. (b) GFP-actin is present in both actin filaments and the soluble actin monomer pool. (Top) GFP-actin colocalizes with filamentous actin as stained by phalloidin; in addition, monomeric GFP-actin is apparent as a diffuse signal. (Bottom) Extraction leaves behind only filamentous actin. (c) Sample time–lapse video microscopy frames, showing paired phase and fluorescent images of GFP-actin MDCK cells infected with L. monocytogenes. The upper cell is not expressing GFP-actin at detectable levels, expression levels vary significantly from cell to cell. (First panel) Black arrowhead, comet tail associated with a moving cytoplasmic bacterium; white arrowheads, extracellular protrusions, which move erratically in and out of focus. Bar, 10 μm. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F1/DC1 (d) Distribution of average bacterial velocities in GFP-actin MDCK cells (n = 48; 2,855 points tracked).
Figure 1
MDCK clones expressing GFP-actin. (a) Western blot stained for actin showing ratio of GFP-actin to actin in the GFP-actin MDCK clone. The upper blot was exposed for 10 s; the lower overexposed for 20 min to enable detection of GFP-actin. The ratio of actin to GFP-actin is ∼50:1. Note that GFP-actin is present in both the Triton X-100–soluble and –insoluble fractions though slightly more is soluble compared with wild-type actin. (b) GFP-actin is present in both actin filaments and the soluble actin monomer pool. (Top) GFP-actin colocalizes with filamentous actin as stained by phalloidin; in addition, monomeric GFP-actin is apparent as a diffuse signal. (Bottom) Extraction leaves behind only filamentous actin. (c) Sample time–lapse video microscopy frames, showing paired phase and fluorescent images of GFP-actin MDCK cells infected with L. monocytogenes. The upper cell is not expressing GFP-actin at detectable levels, expression levels vary significantly from cell to cell. (First panel) Black arrowhead, comet tail associated with a moving cytoplasmic bacterium; white arrowheads, extracellular protrusions, which move erratically in and out of focus. Bar, 10 μm. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F1/DC1 (d) Distribution of average bacterial velocities in GFP-actin MDCK cells (n = 48; 2,855 points tracked).
Figure 1
MDCK clones expressing GFP-actin. (a) Western blot stained for actin showing ratio of GFP-actin to actin in the GFP-actin MDCK clone. The upper blot was exposed for 10 s; the lower overexposed for 20 min to enable detection of GFP-actin. The ratio of actin to GFP-actin is ∼50:1. Note that GFP-actin is present in both the Triton X-100–soluble and –insoluble fractions though slightly more is soluble compared with wild-type actin. (b) GFP-actin is present in both actin filaments and the soluble actin monomer pool. (Top) GFP-actin colocalizes with filamentous actin as stained by phalloidin; in addition, monomeric GFP-actin is apparent as a diffuse signal. (Bottom) Extraction leaves behind only filamentous actin. (c) Sample time–lapse video microscopy frames, showing paired phase and fluorescent images of GFP-actin MDCK cells infected with L. monocytogenes. The upper cell is not expressing GFP-actin at detectable levels, expression levels vary significantly from cell to cell. (First panel) Black arrowhead, comet tail associated with a moving cytoplasmic bacterium; white arrowheads, extracellular protrusions, which move erratically in and out of focus. Bar, 10 μm. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F1/DC1 (d) Distribution of average bacterial velocities in GFP-actin MDCK cells (n = 48; 2,855 points tracked).
Figure 1
MDCK clones expressing GFP-actin. (a) Western blot stained for actin showing ratio of GFP-actin to actin in the GFP-actin MDCK clone. The upper blot was exposed for 10 s; the lower overexposed for 20 min to enable detection of GFP-actin. The ratio of actin to GFP-actin is ∼50:1. Note that GFP-actin is present in both the Triton X-100–soluble and –insoluble fractions though slightly more is soluble compared with wild-type actin. (b) GFP-actin is present in both actin filaments and the soluble actin monomer pool. (Top) GFP-actin colocalizes with filamentous actin as stained by phalloidin; in addition, monomeric GFP-actin is apparent as a diffuse signal. (Bottom) Extraction leaves behind only filamentous actin. (c) Sample time–lapse video microscopy frames, showing paired phase and fluorescent images of GFP-actin MDCK cells infected with L. monocytogenes. The upper cell is not expressing GFP-actin at detectable levels, expression levels vary significantly from cell to cell. (First panel) Black arrowhead, comet tail associated with a moving cytoplasmic bacterium; white arrowheads, extracellular protrusions, which move erratically in and out of focus. Bar, 10 μm. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F1/DC1 (d) Distribution of average bacterial velocities in GFP-actin MDCK cells (n = 48; 2,855 points tracked).
Figure 2
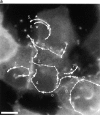
E-cadherin localizes to protrusions but is not required for protrusion structure. Deconvolved sections of fixed cells stained for E-cadherin (a, c, and e) and GFP-actin (b, d, and f). White arrowheads indicate positions of bacteria. (a and b) Two intercellular protrusions. (c and d) Two extracellular protrusions which contain a few E-cadherin puncta. The left-hand protrusion, which contains fewer puncta, passes through several focal planes; only one plane is shown. (e and f) Extracellular protrusions without E-cadherin are morphologically indistinguishable from those with E-cadherin. Bar, 2 μm.
Figure 3
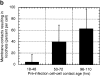
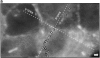
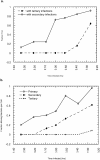
Protrusion likelihood is dependent on host cell. (a) Bacterial paths in a confluent monolayer aged ∼72 h and infected ∼10 h. GFP-actin is expressed at undetectable levels in some cells. Only a fraction of bacterial paths are shown. Arrows denote direction, o's indicate ricochet sites, and asterisks (*) mark protrusions. Dots indicate position with respect to time (20 s intervals). Note that bacteria no. 2 and no. 3 perform both a ricochet and, minutes later, a protrusion, demonstrating that absence of protrusion is not due to a bacterial deficiency. (b) Dependence of ricochet likelihood on monolayer age (≥22 cells, each containing ≥2 bacteria, for each condition). Error bars are SD. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F3/DC1 Bar, 10 μm.
Figure 3
Protrusion likelihood is dependent on host cell. (a) Bacterial paths in a confluent monolayer aged ∼72 h and infected ∼10 h. GFP-actin is expressed at undetectable levels in some cells. Only a fraction of bacterial paths are shown. Arrows denote direction, o's indicate ricochet sites, and asterisks (*) mark protrusions. Dots indicate position with respect to time (20 s intervals). Note that bacteria no. 2 and no. 3 perform both a ricochet and, minutes later, a protrusion, demonstrating that absence of protrusion is not due to a bacterial deficiency. (b) Dependence of ricochet likelihood on monolayer age (≥22 cells, each containing ≥2 bacteria, for each condition). Error bars are SD. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F3/DC1 Bar, 10 μm.
Figure 4
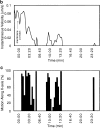
Protrusions undergo bacterially directed fitful movement. (a) White dots indicate bacterial path with respect to time (each dot, 25 s). In this frame, the bacterium is located by the third dot from the top of the image. It slows down as it crosses the plasma membrane at the fourth dot, forming a protrusion. Bar, 2 μm. (b) Initially, the protrusion extends relatively rapidly, then hesitates for ∼2 min and recovers fitful movement for about 4 min before ceasing motility entirely. (c) For each step the bacterium takes, most of the motility is directed along the initial axis of protrusion. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F4/DC1
Figure 4
Protrusions undergo bacterially directed fitful movement. (a) White dots indicate bacterial path with respect to time (each dot, 25 s). In this frame, the bacterium is located by the third dot from the top of the image. It slows down as it crosses the plasma membrane at the fourth dot, forming a protrusion. Bar, 2 μm. (b) Initially, the protrusion extends relatively rapidly, then hesitates for ∼2 min and recovers fitful movement for about 4 min before ceasing motility entirely. (c) For each step the bacterium takes, most of the motility is directed along the initial axis of protrusion. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F4/DC1
Figure 5
Extracellular protrusions exhibit fitful movement. Paths are traced in the adjacent boxes for 5–10 min (each dot = 10 s). (a) Infected cell incubated in the absence of methyl cellulose. Extracellular protrusions appear stubby and thick and move rapidly through many focal planes. (b) Infected cell incubated in 0.5% methyl cellulose, which inhibits Brownian motion. Protrusions are long and slender and are less likely to move out of focus, exhibiting directed motility along the protrusion axis. No new protrusions were formed during either observation. Bar, 10 μm.
Figure 6
Protrusion geometry change. A protrusion extending from an GFP-actin expressing cell into a cell expressing undetectable levels of GFP-actin. (a) The protrusion is originally oblong and clearly connected to the donor cell by a slender, actin-positive stalk, in which it remains for an average time of 35 min. (b) It collapses >150 s to a roughly spherical vacuole surrounding the bacterium. Bar, 5 μm. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F6/DC1
Figure 7
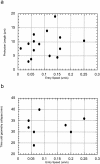
Protrusion length and time to collapse are host dependent. No correlation exists between entry speed and (a) maximum protrusion length and (b) time until protrusion collapse, suggesting that these variables are dependent on the recipient host cell rather than bacterial activity.
Figure 8
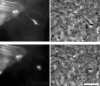
Direct observation of vacuolar lysis. (a) A mixed population of cells, some of which are membrane labeled and/or expressing GFP-actin, were observed by simultaneous video microscopy in two fluorescent channels and by phase contrast. A bacterium moved from the lower right cell (with both actin and membrane labeled) into a protrusion into the center (unlabeled) cell. The white box shows the portion of the field in b and c. Bacterial position is indicated by arrows. (b) The double membrane vacuole is apparent in all three channels. (c) 30 s later, the membrane label is gone (third panel), and phase contrast reveals a morphological change from the spherical vacuole to the free, rod-shaped bacterium. A decrease in GFP-actin fluorescence is observed, but the bacterium remains in focus. Bars, 4 μm. Video available at http://www.jcb.org/cgi/content/full/146/6/1333/F8/DC1 (d) In this example (as in 37% of cases examined), membrane dissolution is immediately preceded by a flash of GFP-actin intensity ∼2.5 min after the protrusion collapses to a roughly spherical geometry (at t = 0:00 min). Videos available at http://www.jcb.org/cgi/content/full/146/6/1333/F8/DC1
Figure 9
ActA is proteolytically cleaved upon acidification in an Mpl-dependent manner. J774 cells were infected with the wild-type strain 10403S (lanes 1–3), a mpl mutant (lanes 4–6) and an actA mutant (lanes 7–9). Cells were pulse-labeled with 35S-methionine and chased, and the medium was replaced with a pH 6.5 buffer (for more details see Materials and Methods). In some cases nigericin was added (lanes 3, 6, and 9) to equilibrate pH across all membranes. Position of molecular mass markers is indicated at the left.
Figure 11
Two examples of TRITC-labeled vacuoles in unlabeled cells adjacent to TRITC-labeled cells. The fluorescent image shows the vacuoles (white arrows), and the corresponding phase contrast image is shown below. Bar, 10 μm.
Figure 10
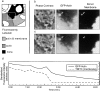
Infectious foci growth and acquisition of motility. (a) Analysis of infectious foci over time reveals that significant intercellular spread to secondary cells occurs between 4 and 5 h. Tertiary cells (two cells removed from the primary cell) begin to be infected at ∼7 h p.i. (b) Increase in the number of motile bacteria occurs before intercellular spread in both primary and secondary cells.
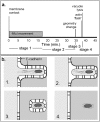
Figure 12
Timeline and model for protrusion uptake. (a) Timeline of a typical bacterium spreading from one cell to another. The initial period of bacterially directed fitful movement lasts ∼15 min and is followed by ∼20 min of stillness, ended by the sudden collapse of the protrusion to a roughly spherical geometry. 3–5 min later, the membrane signal is abrogated, marking vacuole lysis, which is sometimes preceded by a flash of intensity in GFP-actin. (b, 1) The bacterial protrusion, stabilized by E-cadherin, has access to donor cell ATP, and undergoes fitful movement. (2) Movement stops. Tail filaments are partially stabilized by contact with plasma membrane-cytoskeletal linking proteins. (3) Vacuole is formed and acidified, activating PC-PLC and LLO. (4) Membrane is lysed, immediately preceded by actin flash. Remaining filaments are stable and division must occur before movement can resume.
Similar articles
- The Metalloprotease Mpl Supports Listeria monocytogenes Dissemination through Resolution of Membrane Protrusions into Vacuoles.
Alvarez DE, Agaisse H. Alvarez DE, et al. Infect Immun. 2016 May 24;84(6):1806-1814. doi: 10.1128/IAI.00130-16. Print 2016 Jun. Infect Immun. 2016. PMID: 27068088 Free PMC article. - Listeria monocytogenes exploits efferocytosis to promote cell-to-cell spread.
Czuczman MA, Fattouh R, van Rijn JM, Canadien V, Osborne S, Muise AM, Kuchroo VK, Higgins DE, Brumell JH. Czuczman MA, et al. Nature. 2014 May 8;509(7499):230-4. doi: 10.1038/nature13168. Epub 2014 Apr 13. Nature. 2014. PMID: 24739967 Free PMC article. - Evidence implicating the 5' untranslated region of Listeria monocytogenes actA in the regulation of bacterial actin-based motility.
Wong KK, Bouwer HG, Freitag NE. Wong KK, et al. Cell Microbiol. 2004 Feb;6(2):155-66. doi: 10.1046/j.1462-5822.2003.00348.x. Cell Microbiol. 2004. PMID: 14706101 - Bacterial spread from cell to cell: beyond actin-based motility.
Kuehl CJ, Dragoi AM, Talman A, Agaisse H. Kuehl CJ, et al. Trends Microbiol. 2015 Sep;23(9):558-66. doi: 10.1016/j.tim.2015.04.010. Epub 2015 May 25. Trends Microbiol. 2015. PMID: 26021574 Free PMC article. Review. - ActA of Listeria monocytogenes and Its Manifold Activities as an Important Listerial Virulence Factor.
Pillich H, Puri M, Chakraborty T. Pillich H, et al. Curr Top Microbiol Immunol. 2017;399:113-132. doi: 10.1007/82_2016_30. Curr Top Microbiol Immunol. 2017. PMID: 27726006 Review.
Cited by
- Endothelial Cells Use a Formin-Dependent Phagocytosis-Like Process to Internalize the Bacterium Listeria monocytogenes.
Rengarajan M, Hayer A, Theriot JA. Rengarajan M, et al. PLoS Pathog. 2016 May 6;12(5):e1005603. doi: 10.1371/journal.ppat.1005603. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27152864 Free PMC article. - Studying cytoskeletal dynamics in living cells using green fluorescent protein.
Yoon Y, Pitts K, McNiven M. Yoon Y, et al. Mol Biotechnol. 2002 Jul;21(3):241-50. doi: 10.1385/MB:21:3:241. Mol Biotechnol. 2002. PMID: 12102548 Review. - Extracellular vesicles: structure, function, and potential clinical uses in renal diseases.
Borges FT, Reis LA, Schor N. Borges FT, et al. Braz J Med Biol Res. 2013 Oct;46(10):824-30. doi: 10.1590/1414-431X20132964. Epub 2013 Oct 2. Braz J Med Biol Res. 2013. PMID: 24141609 Free PMC article. Review. - A 3-D cell culture system to study epithelia functions using microcarriers.
Jakob PH, Kehrer J, Flood P, Wiegel C, Haselmann U, Meissner M, Stelzer EH, Reynaud EG. Jakob PH, et al. Cytotechnology. 2016 Oct;68(5):1813-25. doi: 10.1007/s10616-015-9935-0. Epub 2016 Feb 4. Cytotechnology. 2016. PMID: 26847791 Free PMC article. - Repeated cycles of rapid actin assembly and disassembly on epithelial cell phagosomes.
Yam PT, Theriot JA. Yam PT, et al. Mol Biol Cell. 2004 Dec;15(12):5647-58. doi: 10.1091/mbc.e04-06-0509. Epub 2004 Sep 29. Mol Biol Cell. 2004. PMID: 15456901 Free PMC article.