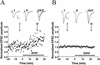Synaptic reliability correlates with reduced susceptibility to synaptic potentiation by brain-derived neurotrophic factor - PubMed (original) (raw)
. 1999 May-Jun;6(3):232-42.
Affiliations
- PMID: 10492005
- PMCID: PMC311306
Synaptic reliability correlates with reduced susceptibility to synaptic potentiation by brain-derived neurotrophic factor
B Berninger et al. Learn Mem. 1999 May-Jun.
Abstract
Recent studies have implicated brain-derived neurotrophic factor (BDNF) in use-dependent modification of hippocampal synapses. BDNF can rapidly potentiate synaptic transmission at glutamatergic synapses by enhancing transmitter release. Using simultaneous perforated patch recording from pairs and triplets of glutamatergic hippocampal neurons, we have examined how the initial state of the glutamatergic synapse determines its susceptibility to synaptic modification by BDNF. We found that the degree of synaptic potentiation by BDNF depends on the initial reliability and strength of the synapse: Relatively weak connections were strongly potentiated, whereas the effect was markedly reduced at stronger synapses. The degree of BDNF-induced potentiation strongly correlated with the initial coefficient of variation (CV) of the amplitude of excitatory postsynaptic currents (EPSCs) and inversely correlated with the initial paired-pulse facilitation, suggesting that synapses with lower release probability (Pr) are more susceptible to the action of BDNF. To determine whether saturation of Pr could have masked the potentiation effect of BDNF in the stronger synapses, we lowered the initial Pr either by reducing the extracellular Ca2+ concentration ([Ca2+]o) or by bath application of adenosine. Synapses that were initially strong remained unaffected by BDNF under these conditions of reduced Pr. Thus, the lack of BDNF effect on synaptic efficacy cannot simply be accounted for by saturation of Pr, but rather may be due to intrinsic changes associated with synaptic maturation that might covary with Pr. Finally, the dependence on initial synaptic strength was also found for divergent outputs of the same presynaptic neuron, suggesting that synaptic terminals with different degrees of responsiveness to BDNF can coexist within in the same neuron.
Figures
Figure 1
Potentiation of glutamatergic transmission by BDNF depends on the initial synaptic strength. (A) Example of a recording of glutamatergic synaptic transmission with a low initial EPSC amplitude (83 pA) prior to exposure to BDNF. Both the designated pre- and postsynaptic neurons were glutamatergic, as judged from the reversal potentials of the synaptic currents recorded in response to stimulation of each neuron in the pair. The presynaptic neuron was stimulated with paired–pulses (50 msec apart) once every 15 sec. Each data point depicts the peak amplitude of the first EPSC normalized to the mean amplitude recorded during the control period (prior to BDNF treatment, dotted line). Duration of exposure to BDNF (100 ng/ml) is depicted by the thick line below. Shown above are sample traces (averages of 10 events) recorded at the time marked by I and II. Scales, 40 pA, 10 msec. (B) Example of a recording of glutamatergic synaptic transmission onto another glutamatergic neuron with a large initial amplitude (1034 pA) prior to exposure to BDNF. Scales, 200 pA, 10 msec.
Figure 2
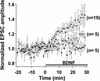
Dose dependence of the potentiation effect of BDNF. Average time course of synaptic potentiation following exposure to 0 (♦), 20 (□), and 100 (▴) ng/ml of BDNF. Prior to averaging, EPSC amplitudes from each experiment were normalized to the mean amplitude recorded during control period (before treatment). Data points represent mean ±
s.e.m.
n refers to the number of experiments.
Figure 3
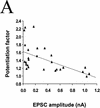
The degree of synaptic potentiation by BDNF correlates with initial synaptic strength, CV, and paired–pulse facilitation. (A) Relationship between synaptic potentiation and initial EPSC amplitude. Initial EPSC amplitude was calculated for 40 events during 10 min of control period. The potentiation factor is defined as the ratio of mean EPSC amplitude during the last 10 min of the experiment following BDNF treatment to that during the control period. Each point refers to the result of one experiment. The line represents the best linear fit of the data (r = −0.552, P = 0.0028, ANOVA). (B) Relationship between synaptic potentiation and the initial CV. Initial CV was determined as ς/mean, in which ς and mean are the standard deviation and mean value of the EPSC amplitude during the control period. The line represents the best linear fit of the data (r = 0.741, P < 0.0001, ANOVA). (C) Relationship between synaptic potentiation and initial paired–pulse facilitation. The paired–pulse ratio is the ratio of the amplitude of the second EPSC to that of the first. The initial paired–pulse ratio was computed as the average value observed during the control period. The line represents the best linear fit of the data (r = 0.586, P = 0.0042, ANOVA).
Figure 3
The degree of synaptic potentiation by BDNF correlates with initial synaptic strength, CV, and paired–pulse facilitation. (A) Relationship between synaptic potentiation and initial EPSC amplitude. Initial EPSC amplitude was calculated for 40 events during 10 min of control period. The potentiation factor is defined as the ratio of mean EPSC amplitude during the last 10 min of the experiment following BDNF treatment to that during the control period. Each point refers to the result of one experiment. The line represents the best linear fit of the data (r = −0.552, P = 0.0028, ANOVA). (B) Relationship between synaptic potentiation and the initial CV. Initial CV was determined as ς/mean, in which ς and mean are the standard deviation and mean value of the EPSC amplitude during the control period. The line represents the best linear fit of the data (r = 0.741, P < 0.0001, ANOVA). (C) Relationship between synaptic potentiation and initial paired–pulse facilitation. The paired–pulse ratio is the ratio of the amplitude of the second EPSC to that of the first. The initial paired–pulse ratio was computed as the average value observed during the control period. The line represents the best linear fit of the data (r = 0.586, P = 0.0042, ANOVA).
Figure 3
The degree of synaptic potentiation by BDNF correlates with initial synaptic strength, CV, and paired–pulse facilitation. (A) Relationship between synaptic potentiation and initial EPSC amplitude. Initial EPSC amplitude was calculated for 40 events during 10 min of control period. The potentiation factor is defined as the ratio of mean EPSC amplitude during the last 10 min of the experiment following BDNF treatment to that during the control period. Each point refers to the result of one experiment. The line represents the best linear fit of the data (r = −0.552, P = 0.0028, ANOVA). (B) Relationship between synaptic potentiation and the initial CV. Initial CV was determined as ς/mean, in which ς and mean are the standard deviation and mean value of the EPSC amplitude during the control period. The line represents the best linear fit of the data (r = 0.741, P < 0.0001, ANOVA). (C) Relationship between synaptic potentiation and initial paired–pulse facilitation. The paired–pulse ratio is the ratio of the amplitude of the second EPSC to that of the first. The initial paired–pulse ratio was computed as the average value observed during the control period. The line represents the best linear fit of the data (r = 0.586, P = 0.0042, ANOVA).
Figure 4
Lowering Pr at stronger synapses does not unmask response to BDNF. (A) Example for absence of BDNF effect after lowering Pr by reducing [Ca2+]o. Initial EPSC was ∼−1050 pA and was reduced to −30 pA after reducing [Ca2+]o from 3 to 0.5 m
m
and elevating [Mg2+]o from 2 to 3 m
m
. The vertical line depicts the time point following lowering [Ca2+]o, after which EPSCs amplitudes were normalized to the new mean value recorded during the last 10 min prior to exposure to BDNF (100 ng/ml). Shown at top are sample traces of EPSCs (average of 10 events). Scales, 200 pA, 10 msec (left two traces), and 10 pA, 10 msec (right three traces). Duration of low [Ca2+]o and BDNF treatments are indicated by the thick lines below. (*) Monosynaptic EPSCs monitored. Note that polysynaptic currents disappeared after the low [Ca2+]o treatment. (B) Summary of results from experiments in which Pr was reduced by either treatment with low [Ca2+]o or with adenosine. Potentiation factor observed after the BDNF treatment was defined as in Fig. 3. Bars represent average values obtained from all experiments on weak synapses (EPSC amplitude <600 pA) and strong synapses (>800 pA) in the normal recording medium as well as experiments on strong synapses (>800 pA) in the presence of low [Ca2+]o and adenosine. The average EPSC amplitude before and after the low [Ca2+]o or adenosine treatment is shown at bottom. (N.A.), Not applicable. Error bars,
s.e.m.
Figure 5
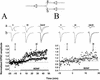
Differential synaptic potentiation by BDNF at divergent outputs of a single presynaptic neuron. (A, B) Two examples of triplet recordings in which a single presynaptic glutamatergic neuron projected onto two glutamatergic neurons (see diagram at top), with differing synaptic initial synaptic strength. Each data point represents the peak amplitude of the EPSC, normalized to the mean amplitude during the control period (prior to BDNF treatment), as indicated by the dotted line. Filled and open symbols depict EPSCs for initially relatively weak and strong connections, respectively. Shown at top are sample traces (averages of 10 events) at the time indicated. Scales, 200 pA, 5 msec (A) and 50 pA, 5 msec (B). Initial amplitudes of the EPSCs were 460 and 1010 pA in A and 42 and 336 pA in B.
Similar articles
- Chronic BDNF deficiency permanently modifies excitatory synapses in the piriform cortex.
Nanobashvili A, Jakubs K, Kokaia M. Nanobashvili A, et al. J Neurosci Res. 2005 Sep 1;81(5):696-705. doi: 10.1002/jnr.20578. J Neurosci Res. 2005. PMID: 16035106 - Impaired transmission at corticothalamic excitatory inputs and intrathalamic GABAergic synapses in the ventrobasal thalamus of heterozygous BDNF knockout mice.
Laudes T, Meis S, Munsch T, Lessmann V. Laudes T, et al. Neuroscience. 2012 Oct 11;222:215-27. doi: 10.1016/j.neuroscience.2012.07.005. Epub 2012 Jul 13. Neuroscience. 2012. PMID: 22796079 - Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS.
Lessmann V. Lessmann V. Gen Pharmacol. 1998 Nov;31(5):667-74. doi: 10.1016/s0306-3623(98)00190-6. Gen Pharmacol. 1998. PMID: 9809461 Review. - BDNF signaling in the formation, maturation and plasticity of glutamatergic and GABAergic synapses.
Gottmann K, Mittmann T, Lessmann V. Gottmann K, et al. Exp Brain Res. 2009 Dec;199(3-4):203-34. doi: 10.1007/s00221-009-1994-z. Epub 2009 Sep 24. Exp Brain Res. 2009. PMID: 19777221 Review.
Cited by
- Intracellular Ca2+ stores and Ca2+ influx are both required for BDNF to rapidly increase quantal vesicular transmitter release.
Amaral MD, Pozzo-Miller L. Amaral MD, et al. Neural Plast. 2012;2012:203536. doi: 10.1155/2012/203536. Epub 2012 Jul 3. Neural Plast. 2012. PMID: 22811938 Free PMC article. - Brain-derived neurotrophic factor controls activity-dependent maturation of CA1 synapses by downregulating tonic activation of presynaptic kainate receptors.
Sallert M, Rantamäki T, Vesikansa A, Anthoni H, Harju K, Yli-Kauhaluoma J, Taira T, Castren E, Lauri SE. Sallert M, et al. J Neurosci. 2009 Sep 9;29(36):11294-303. doi: 10.1523/JNEUROSCI.0560-09.2009. J Neurosci. 2009. PMID: 19741136 Free PMC article. - Single-cell characterization of retrograde signaling by brain-derived neurotrophic factor.
Magby JP, Bi C, Chen ZY, Lee FS, Plummer MR. Magby JP, et al. J Neurosci. 2006 Dec 27;26(52):13531-6. doi: 10.1523/JNEUROSCI.4576-06.2006. J Neurosci. 2006. PMID: 17192436 Free PMC article. - Protective Effects of Cornel Iridoid Glycoside in Rats After Traumatic Brain Injury.
Ma D, Wang N, Fan X, Zhang L, Luo Y, Huang R, Zhang L, Li Y, Zhao G, Li L. Ma D, et al. Neurochem Res. 2018 Apr;43(4):959-971. doi: 10.1007/s11064-018-2501-3. Epub 2018 Feb 28. Neurochem Res. 2018. PMID: 29492766 - Brain-derived neurotrophic factor modulates cerebellar plasticity and synaptic ultrastructure.
Carter AR, Chen C, Schwartz PM, Segal RA. Carter AR, et al. J Neurosci. 2002 Feb 15;22(4):1316-27. doi: 10.1523/JNEUROSCI.22-04-01316.2002. J Neurosci. 2002. PMID: 11850459 Free PMC article.
References
- Berninger B, Poo M-m. Fast actions of neurotrophic factors. Curr Opin Neurobiol. 1996;6:324–330. - PubMed
- Blöchl A, Thoenen H. Characterization of nerve growth factor (NGF) release from hippocampal neurons: Evidence for a constitutive and an unconventional sodium-dependent regulated pathway. Eur J Neurosci. 1995;7:1220–1228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous