Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission - PubMed (original) (raw)
. 1999 Oct 1;19(19):8377-88.
doi: 10.1523/JNEUROSCI.19-19-08377.1999.
R Kanjhan, N P Raybould, D Greenwood, S G Salih, L Järlebark, L D Burton, V C Setz, M B Cannell, C Soeller, D L Christie, S Usami, A Matsubara, H Yoshie, A F Ryan, P R Thorne
Affiliations
- PMID: 10493739
- PMCID: PMC6783052
- DOI: 10.1523/JNEUROSCI.19-19-08377.1999
Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission
G D Housley et al. J Neurosci. 1999.
Abstract
Extracellular ATP has multimodal actions in the cochlea affecting hearing sensitivity. ATP-gated ion channels involved in this process were characterized in the guinea pig cochlea. Voltage-clamped hair cells exhibited a P2 receptor pharmacology compatible with the assembly of ATP-gated ion channels from P2X(2) receptor subunits. Reverse transcription-PCR experiments confirmed expression of the P2X(2-1) receptor subunit mRNA isoform in the sensory epithelium (organ of Corti); a splice variant that confers desensitization, P2X(2-2), was the predominant subunit isoform expressed by primary auditory neurons. Expression of the ATP-gated ion channel protein was localized using a P2X(2) receptor subunit-specific antiserum. The highest density of P2X(2) subunit-like immunoreactivity in the cochlea occurred on the hair cell stereocilia, which faces the endolymph. Tissues lining this compartment exhibited significant P2X(2) receptor subunit expression, with the exception of the stria vascularis. Expression of ATP-gated ion channels at these sites provides a pathway for the observed ATP-induced reduction in endocochlear potential and likely serves a protective role, decoupling the "cochlear amplifier" in response to stressors, such as noise and ischemia. Within the perilymphatic compartment, immunolabeling on Deiters' cells is compatible with purinergic modulation of cochlear micromechanics. P2X(2) receptor subunit expression was also detected in spiral ganglion primary afferent neurons, and immunoelectron microscopy localized these subunits to postsynaptic junctions at both inner and outer hair cells. The former supports a cotransmitter role for ATP in a subset of type I spiral ganglion neurons, and latter represents the first characterization of a receptor for a fast neurotransmitter associated with the type II spiral ganglion neurons.
Figures
Fig. 1.
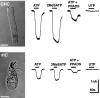
Whole-cell voltage-clamp analysis of P2X receptor pharmacology of cochlear hair cells. ATP and 2MeSATP (10 μ
m
) gave sustained inward current responses. PPADS (10 μ
m
) blocked this effect. The cells were unresponsive to UTP (100 μ
m
). Holding potential, −60 mV. Scale bars, 12 μm.
Fig. 2.
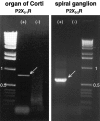
Molecular characterization of P2X2receptor expression in sensory and neural epithelium. Agarose gels showing an 803 bp P2X2–1 receptor isoform RT-PCR product derived from organ of Corti mRNA template and a 596 bp RT-PCR product (P2X2–2 receptor isoform) derived from spiral ganglion mRNA. +, Indicates reverse-transcribed mRNA; −, indicates omission of the MMLV reverse transcriptase.
Fig. 3.
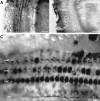
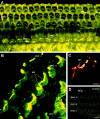
Identification of P2X2receptor protein expression in the organ of Corti. A, Whole-mount surface preparation of turn three of the guinea pig organ of Corti using immunoperoxidase histochemistry. Note the intense P2X2 receptor-immunopositive labeling of the three rows of OHC and the single row of IHC. B, Control for specificity of the P2X2R96ab antiserum was demonstrated by block of the immunolabeling by preadsorption with the target 18 amino acid peptide (10 μg/ml). A and B were adjacent regions of organ of Corti tissue (P2X2R96ab antiserum, 1:2000). C, Detail of third turn organ of Corti region showing that the most intense P2X2 receptor immunolabeling occurred on the stereocilia. Note the lack of immunolabeling on the endolymphatic surfaces of the Deiters’ cell processes (DCp), except for row 3(P2X2R96ab antiserum, 1:2000). Scale bars:A, B, 50 μm; C, 15 μm.
Fig. 4.
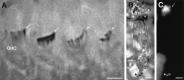
Localization of P2X2 receptor subunits on hair cell stereocilia. A, Detail of P2X2 receptor immunolabeling of row 2 OHC stereocilia from the third turn of the cochlea (arrows) (P2X2R96ab antiserum, 1:4000). B,C, Light-field and immunofluorescence images, respectively, of an isolated guinea pig OHC showing binding of avidin-linked FITC microspheres to the stereocilia (arrows) (P2X2R96ab antiserum, 1:20). Labeling is also apparent on processes attached to the base of the OHC, which likely correspond to synaptic terminals (*). Scale bars, 5 μm.
Fig. 5.
P2X2 receptor immunofluorescence labeling of the organ of Corti imaged by stereo-confocal reconstruction. A, Red–green_stereo-image derived from 25 confocal sections spanning 15 μm from the tips of the hair cell stereocilia (stc) in turn one organ of Corti. Note that the apical-most region of the OHC stereocilia shows the greatest P2X2 receptor immunofluorescence, with little labeling on the cuticular plates. Labeling of the Deiters’ cell processes (DCp) clearly lies below the hair cell cuticular plate level, within the perilymphatic space. The lack of labeling of the reticular lamina aspect of the pillar cells permits a view into the tunnel of Corti (TC). The endolymphatic surface of the IHC, particularly the stereocilia, show immunolabeling. Inner sulcus cells,is. B, Stereo reconstruction of immunolabeling on the OHC stereocilia (rows 1–3) showing that the brightest immunofluorescence occurs at the tip region.C, Confocal optical section (0.5 μm) of P2X2 receptor immunofluorescence at high magnification through the tip region of the stereocilia of an OHC. Note that the P2X2 receptor immunolabeling is on the outside of individual stereocilia (stc, arrow). Both_B and C are from turn three of the organ of Corti. D is from the turn one to two region.D, Control confocal immunofluorescence image of organ of Corti tissue (omission of P2X2R96ab antiserum; secondary antibody, Cy3-conjugated goat anti-rabbit IgG, 1:500). Scale bars:A, 10 μm; B, C, 4 μm;D, 10 μm.
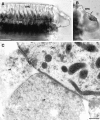
Fig. 6.
Electron microscopic immunolocalization of the P2X2 receptor protein to the stereocilia of the guinea pig OHC. Immunogold labeling (15 nm particles) were detected at greatest density in association with the membrane of the OHC stereocilia (stc; arrows). Immunolabeling on the endolymphatic aspect of the cuticular plate (OHC-cp) was minimal, as was the labeling on the adjacent Deiters’ cell process (DCp)._Inse_t shows P2X2 receptor immunolabeling in a cross section through the stereocilia. Scale bars: 500 nm;inset, 1 μm.
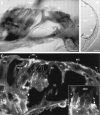
Fig. 7.
P2X2 receptor immunolabeling of radial sections of the cochlea. A, P2X2receptor immunoperoxidase labeling of a radial section of organ of Corti (30 μm cryosection; P2X2R96ab antiserum, 1:2000). OHC are unlabeled apart from the stereocilia (stc). Deiters’ cells (DC) and their processes show immunolabeling, as does the synaptic region adjacent to the IHC. Pillar cell, PC; crossing fibers, cf.B, Immunoperoxidase labeling of the spiral ligament. Note the P2X2 receptor expression in the external sulcus cell (es), root cell region leading to the spiral prominence (sp), whereas the stria vascularis (sv) does not express these ATP-gated ion channel subunits (Reissner’s membrane, rm; scala media,SM). C, Two-photon immunofluorescence optical section (0.9 μm) showing strong P2X2 receptor immunolabeling at a number of sites in a radial section through the organ of Corti. Detail of the labeling in the Deiters’ cell (DC) cup region is shown in the inset [P2X2R96ab antiserum, 1:400; secondary antiserum (Alexa 488-conjugated goat antirabbit IgG), 1:400; 35 μm cryosection]. Deiters’ cell process, DCp; Hensen’s cell,HC; inner sulcus cell, is; tunnel of Corti, TC. Scale bars: A, 20 μm;B, 50 μm; C, 20 μm;inset, 5 μm.
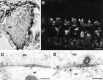
Fig. 8.
Localization of P2X2 receptor expression associated with the sensory region of the OHC.A, Immunoperoxidase labeling of a whole-mount preparation of OHC and associated Deiters’ cells (DC). Note that, although the body of the OHC is unlabeled, punctate staining is apparent at the base of the OHC (arrows) in addition to the more diffuse immunostaining of the Deiters’ cells.B, Detail of the OHC boxed as out of focus in_A_, which has detached from the body of cells and clearly demonstrates the Deiters’ cells cup region supporting the base of the OHC. Punctate synapse-like immunolabeling is associated with this region (arrow). C, Immunogold labeling of the postsynaptic thickening of the OHC–type II spiral ganglion neuron (afferent) synapse (aff, arrows). An adjacent efferent (eff) synapse is unlabeled. Scale bars: A, B, 20 μm; C, 0.2 μm.
Fig. 9.
Characterization of P2X2receptor expression associated with the auditory afferent–inner hair cell synapse. A, Immunoperoxidase labeling of a subgroup of (principally type I) spiral ganglion neurons (arrows) after permeabilization with Triton X-100 (P2X2R96ab 1:4000). B, Two-photon P2X2 receptor immunofluorescence optical section showing punctate labeling at the level of the IHC synaptic specialization in the turn two to three region [P2X2R96ab, 1:400; secondary antibody (Alexa 488-conjugated goat antirabbit IgG), 1:400]. C,D, Immunogold localization of P2X2 receptor subunits on the postsynaptic thickening of type I spiral ganglion neuron–inner hair cell synapses (arrows).A and B were obtained from 20 μm cryosections. Scale bars: A, 25 μm; B, 10 μm; C, D, 0.2 μm.
Fig. 10.
Reconstruction of P2X2receptor protein expression in the organ of Corti from confocal immunofluorescence. A–C show progressive aspects of a 90 μm3 of organ of Corti from turn one reconstructed using VoxelView software. The reconstructed image has been rotated relative to the imaging plane, which passed down through the hair cells from their stereocilia (stc).B and C show side views of the Deiters’ cells (DC), with their processes (DCp) extending to support the cuticular plate region of the OHC. Note that, apart from the stereocilia (stc) labeling (A) and a few OHC cuticular plates, the rest of the OHC membrane is not labeled. The voxel resolution is 0.75 μm3. Inner sulcus cells, is.D, Views of an isosurface rendering of P2X2receptor protein expression computed from a subset (box_in A) of the serial confocal optical section stack reconstructed in A–C. Stereocilia of two OHC are shown by P2X2 immunofluorescence labeling, although the body of the OHC is unlabeled and therefore not visible. This provides an unparalleled view of the immunolabeling on the associated Deiters’ cell processes extending to the reticular lamina and Deiters’ cell cup regions, which support the base of the OHC (see Fig.7_C, inset). Scale bars:A–C, 10 μm; D, 15 μm.
Similar articles
- ATP-gated ion channels assembled from P2X2 receptor subunits in the mouse cochlea.
Järlebark LE, Housley GD, Raybould NP, Vlajkovic S, Thorne PR. Järlebark LE, et al. Neuroreport. 2002 Oct 28;13(15):1979-84. doi: 10.1097/00001756-200210280-00030. Neuroreport. 2002. PMID: 12395104 - Purinergic regulation of sound transduction and auditory neurotransmission.
Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Järlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Muñoz DJ, Thorne PR. Housley GD, et al. Audiol Neurootol. 2002 Jan-Feb;7(1):55-61. doi: 10.1159/000046865. Audiol Neurootol. 2002. PMID: 11914528 - Immunohistochemical localization of adenosine 5'-triphosphate-gated ion channel P2X(2) receptor subunits in adult and developing rat cochlea.
Järlebark LE, Housley GD, Thorne PR. Järlebark LE, et al. J Comp Neurol. 2000 Jun 5;421(3):289-301. doi: 10.1002/(sici)1096-9861(20000605)421:3<289::aid-cne1>3.0.co;2-0. J Comp Neurol. 2000. PMID: 10813788 - Extracellular nucleotide signaling in the inner ear.
Housley GD. Housley GD. Mol Neurobiol. 1998 Feb;16(1):21-48. doi: 10.1007/BF02740601. Mol Neurobiol. 1998. PMID: 9554700 Review. - Purinergic signaling in the organ of Corti: Potential therapeutic targets of sensorineural hearing losses.
Berekméri E, Szepesy J, Köles L, Zelles T. Berekméri E, et al. Brain Res Bull. 2019 Sep;151:109-118. doi: 10.1016/j.brainresbull.2019.01.029. Epub 2019 Feb 2. Brain Res Bull. 2019. PMID: 30721767 Review.
Cited by
- Research progress in delineating the pathological mechanisms of _GJB2_-related hearing loss.
Wang Y, Jin Y, Zhang Q, Xiong Y, Gu X, Zeng S, Chen W. Wang Y, et al. Front Cell Neurosci. 2023 Jun 2;17:1208406. doi: 10.3389/fncel.2023.1208406. eCollection 2023. Front Cell Neurosci. 2023. PMID: 37333892 Free PMC article. Review. - Effects of Noise Damage on the Purinergic Signal of Cochlear Spiral Ganglion Cells in Guinea Pigs.
Shi M, Cao L, Ding D, Yu W, Lv P, Yu N. Shi M, et al. Mol Biotechnol. 2024 Feb;66(2):321-331. doi: 10.1007/s12033-023-00755-6. Epub 2023 May 5. Mol Biotechnol. 2024. PMID: 37145220 - Identifying targets to prevent aminoglycoside ototoxicity.
Kim J, Hemachandran S, Cheng AG, Ricci AJ. Kim J, et al. Mol Cell Neurosci. 2022 May;120:103722. doi: 10.1016/j.mcn.2022.103722. Epub 2022 Mar 24. Mol Cell Neurosci. 2022. PMID: 35341941 Free PMC article. Review. - In vivo real-time imaging reveals megalin as the aminoglycoside gentamicin transporter into cochlea whose inhibition is otoprotective.
Kim J, Ricci AJ. Kim J, et al. Proc Natl Acad Sci U S A. 2022 Mar 1;119(9):e2117946119. doi: 10.1073/pnas.2117946119. Proc Natl Acad Sci U S A. 2022. PMID: 35197290 Free PMC article. - ATP and ACh Evoked Calcium Transients in the Neonatal Mouse Cochlear and Vestibular Sensory Epithelia.
Rabbitt RD, Holman HA. Rabbitt RD, et al. Front Neurosci. 2021 Sep 8;15:710076. doi: 10.3389/fnins.2021.710076. eCollection 2021. Front Neurosci. 2021. PMID: 34566562 Free PMC article.
References
- Ashmore JF. The G. L. Brown Prize Lecture. The cellular machinery of the cochlea. Exp Physiol. 1994;79:113–134. - PubMed
- Bobbin RP, Thompson MH. Effects of putative transmitters on afferent cochlear transmission. Ann Otol Rhino Laryngol. 1978;87:185–190. - PubMed
- Brake AJ, Wagenbach MJ, Julius D. New structural motif for ligand-gated ion channels defined by an ionotropic ATP receptor. Nature. 1994;371:519–523. - PubMed
- Brändle U, Spielmanns P, Osteroth R, Sim J, Surprenant A, Buell G, Ruppersberg JP, Plinkert PK, Zenner H-P, Glowatzki E. Desensitization of the P2X2 receptor controlled by alternative splicing. FEBS Lett. 1997;404:294–298. - PubMed
- Brown MC, Berglund AM, Kiang NY, Ryugo DK. Central trajectories of type II spiral ganglion neurons. J Comp Neurol. 1988;287:581–590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases