Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes - PubMed (original) (raw)
Matrix metalloproteinase-9/gelatinase B is required for process outgrowth by oligodendrocytes
L Y Oh et al. J Neurosci. 1999.
Abstract
Oligodendrocytes (OLs) extend processes to contact axons as a prerequisite step in myelin formation. As the OL processes migrate toward their axonal targets, they modify adhesion to their substrate, an event that may be regulated by matrix metalloproteinases (MMPs). In the mouse optic nerve, MMP-9/gelatinase B increases during myelin formation. Although tissue inhibitor of metalloproteinase (TIMP)-3 also increases in parallel, the developing optic nerve has focally active MMPs demonstrable by in situ zymography. The distribution of proteolytic activity is similar to that of myelin basic protein, a marker of myelin formation. OLs in culture secrete MMP-9 and express active cell-associated metalloproteinases at the growing tips of their processes. TIMP-1 and a function-perturbing anti-MMP-9 antibody attenuate outgrowth of processes by OLs, indicating a requirement for MMP-9 in process outgrowth. Process reformation is retarded significantly in OLs cultured from MMP-9 null mice, as compared with controls, providing genetic evidence that MMP-9 is necessary for process outgrowth. These data show that MMP-9 facilitates process outgrowth by OLs in vivo and in culture.
Figures
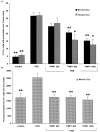
Fig. 1.
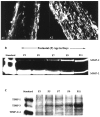
Expression of MMP-2 and MMP-9 during myelination in developing optic nerve. The period of myelin formation in the CD1 mouse optic nerve is shown by MBP immunofluorescence. A1, A2, Weak immunoreactivity of MBP at P7 is shown (A1), whereas strong MBP staining indicates active myelination at P9 (A2). B, Gelatin zymography of optic nerve from P3 to P11 CD1 mice shows a gradual increase in MMP-9 in contrast to the decrease in MMP-2 during this period. C, Reverse gelatin zymography for TIMPs is shown. TIMP-3 increased in mouse optic nerve during the period of myelination (P3 to P11), whereas no significant changes in TIMP-1 or TIMP-2/-4 levels were detected.
Fig. 2.
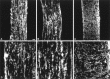
Localization of MMP activity in developing optic nerve in vivo by in situ zymography. A cryostat section of P9 mouse optic nerve shows proteolytic activity of MMPs by in situ zymography (A). Localization of gelatinolytic activity (A) was reduced in the presence of TIMP-1 (500 ng/ml; B) or 1,10-phenanthroline (50 μ
m;
C). Scale bar, 50 μm.
Fig. 3.
Comparison of localization of gelatinase activity with MBP or GFAP immunoreactivity in developing optic nerve. Immunohistochemistry of MBP (A, D) and GFAP (C, F) and in situ zymography (B, E) of P9 mouse optic nerve show that the pattern of proteolytic activity shown by in situ zymography appeared to be similar to that of longitudinal MBP immunoreactivity. In contrast, GFAP immunoreactivity was mainly perpendicular to that of MBP and _in situ_zymography (C, F). Scale bars:A–C, 100 μm;D_–_F, 50 μm.
Fig. 4.
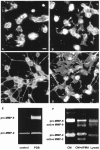
Morphology and MMP-9 expression by OLs. Bovine or murine OLs express MMP-9 and form processes after PDB treatment.A, B, Control untreated bovine (A) and murine (B) OLs are shown. C, D, In response to PDB treatment, both bovine (C) and murine (D) OLs upregulate the extent of their process formation. Scale bar_,_ 10 μm. E, The expression of MMP-9 correlates with the process outgrowth induced by PDB (shown for bovine OLs only). F, The conditioned medium (CM) of OL cultures contains mainly pro-MMP-9, because APMA treatment of the conditioned medium (CM+APMA) converts pro-MMP-9 to the lower molecular weight active MMP-9. However, active MMP-9 was detected in OL cell lysate (Lysate).
Fig. 5.
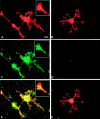
Localization of MMP activity in OLs in culture by_in situ_ zymography. A, _B,_O1+ OLs treated with PDB to induce process formation are shown.C, D, Net proteolytic activity of MMP (C) was localized on OL soma, processes, and the tip of a process (inset of A, C, E), whereas in the presence of 1,10-phenanthroline (50 μ
m
), the MMP activity by OLs was inhibited (D).E, A superimposed image overlapping signal from O1 immunoreactivity (A) and in situ_zymography (C) is shown. F, The corresponding superimposed image of B and_D is shown. Scale bar, 10 μm.
Fig. 6.
Effects of TIMP-1 on process outgrowth by OLs. The process outgrowth induced by 10 n
m
PDB in human and bovine OLs (A) and in mouse OLs (B) was inhibited by recombinant human TIMP-1. TIMP-1 concentrations are given in nanograms per milliliter. *p < 0.01 and **p < 0.001 compared with PDB treatment alone.
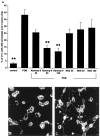
Fig. 7.
Effect of a neutralizing antibody to MMP-9 on process outgrowth by OLs. A, A neutralizing MMP-9 antibody (Abmmp-9) inhibits the PDB-induced process outgrowth by bovine OLs. The concentrations of sheep anti-porcine MMP-9 and preimmune normal sheep serum (NSS) used are in micrograms per milliliter. B, The inhibitory effect of_Abmmp-9_ on OL process outgrowth induced by PDB is shown.C, In the absence of Abmmp-9, OLs extend significant processes in response to PDB. **p < 0.001 compared with PDB treatment alone. Scale bar, 10 μm.
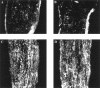
Fig. 8.
Comparable myelin formation during the development of the optic nerve of MMP-9 null mice and their wild-type controls.A, C, Optic nerves from wild-type controls at P7 and P10, respectively. B, D, Optic nerves from MMP-9 null mice at P7 and P10, respectively. MBP immunoreactivity of the optic nerves from MMP-9 null mice suggests that developmental myelination occurs normally in the absence of MMP-9.
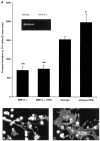
Fig. 9.
Effect of a targeted mutation in MMP-9 on process extension by OLs in culture. A, OLs derived from MMP-9 null mice exhibit a reduced capacity to extend processes compared with OLs from wild-type mice, either under basal culture conditions or in response to 10 n
m
PDB. *p < 0.05 and **p < 0.001 compared with wild type.Inset, A zymogram demonstrates the lack of MMP-9 in MMP-9 null mice compared with that in wild type. B, A photograph of OLs from MMP-9 null mice after 48 hr of PDB treatment is shown. C, Corresponding PDB-treated OLs from wild-type controls are displayed.
Similar articles
- Spatiotemporal expression patterns of metalloproteinases and their inhibitors in the postnatal developing rat cerebellum.
Vaillant C, Didier-Bazès M, Hutter A, Belin MF, Thomasset N. Vaillant C, et al. J Neurosci. 1999 Jun 15;19(12):4994-5004. doi: 10.1523/JNEUROSCI.19-12-04994.1999. J Neurosci. 1999. PMID: 10366632 Free PMC article. - The expression of matrix metalloproteinase-12 by oligodendrocytes regulates their maturation and morphological differentiation.
Larsen PH, Yong VW. Larsen PH, et al. J Neurosci. 2004 Sep 1;24(35):7597-603. doi: 10.1523/JNEUROSCI.2092-04.2004. J Neurosci. 2004. PMID: 15342725 Free PMC article. - Oligodendrocytes utilize a matrix metalloproteinase, MMP-9, to extend processes along an astrocyte extracellular matrix.
Uhm JH, Dooley NP, Oh LY, Yong VW. Uhm JH, et al. Glia. 1998 Jan;22(1):53-63. doi: 10.1002/(sici)1098-1136(199801)22:1<53::aid-glia5>3.0.co;2-9. Glia. 1998. PMID: 9436787 - Matrix metalloproteinases and their inhibitors.
Kugler A. Kugler A. Anticancer Res. 1999 Mar-Apr;19(2C):1589-92. Anticancer Res. 1999. PMID: 10365151 Review. - Mechanisms for pro matrix metalloproteinase activation.
Murphy G, Stanton H, Cowell S, Butler G, Knäuper V, Atkinson S, Gavrilovic J. Murphy G, et al. APMIS. 1999 Jan;107(1):38-44. doi: 10.1111/j.1699-0463.1999.tb01524.x. APMIS. 1999. PMID: 10190278 Review.
Cited by
- Expression of angiogenic and fibrogenic factors in proliferative vitreoretinal disorders.
Abu El-Asrar AM, Van den Steen PE, Al-Amro SA, Missotten L, Opdenakker G, Geboes K. Abu El-Asrar AM, et al. Int Ophthalmol. 2007 Feb;27(1):11-22. doi: 10.1007/s10792-007-9053-x. Epub 2007 Mar 21. Int Ophthalmol. 2007. PMID: 17375263 - Angiotensin II activates matrix metalloproteinase type II and mimics age-associated carotid arterial remodeling in young rats.
Wang M, Zhang J, Spinetti G, Jiang LQ, Monticone R, Zhao D, Cheng L, Krawczyk M, Talan M, Pintus G, Lakatta EG. Wang M, et al. Am J Pathol. 2005 Nov;167(5):1429-42. doi: 10.1016/S0002-9440(10)61229-1. Am J Pathol. 2005. PMID: 16251426 Free PMC article. - Metalloproteinase-dependent predegeneration in vitro enhances axonal regeneration within acellular peripheral nerve grafts.
Krekoski CA, Neubauer D, Graham JB, Muir D. Krekoski CA, et al. J Neurosci. 2002 Dec 1;22(23):10408-15. doi: 10.1523/JNEUROSCI.22-23-10408.2002. J Neurosci. 2002. PMID: 12451140 Free PMC article. - Secretagogin-dependent matrix metalloprotease-2 release from neurons regulates neuroblast migration.
Hanics J, Szodorai E, Tortoriello G, Malenczyk K, Keimpema E, Lubec G, Hevesi Z, Lutz MI, Kozsurek M, Puskár Z, Tóth ZE, Wagner L, Kovács GG, Hökfelt TG, Harkany T, Alpár A. Hanics J, et al. Proc Natl Acad Sci U S A. 2017 Mar 7;114(10):E2006-E2015. doi: 10.1073/pnas.1700662114. Epub 2017 Feb 21. Proc Natl Acad Sci U S A. 2017. PMID: 28223495 Free PMC article. - Remyelination therapy for multiple sclerosis.
Keough MB, Yong VW. Keough MB, et al. Neurotherapeutics. 2013 Jan;10(1):44-54. doi: 10.1007/s13311-012-0152-7. Neurotherapeutics. 2013. PMID: 23070731 Free PMC article. Review.
References
- Althaus HH, Klöppner S, Schmidt-Schultz T, Schwartz P. Nerve growth factor induces proliferation and enhances fiber regeneration in oligodendrocytes isolated from adult pig brain. Neurosci Lett. 1992;135:219–223. - PubMed
- Amberger VR, Avellana-Adalid V, Hensel T, Baron-van EA, Schwab ME. Oligodendrocyte-type 2 astrocyte progenitors use a metalloendoprotease to spread and migrate on CNS myelin. Eur J Neurosci. 1997;9:151–162. - PubMed
- Anthony DC, Ferguson B, Matyzak MK, Miller KM, Esiri MM, Perry VH. Differential matrix metalloproteinase expression in cases of multiple sclerosis and stroke. Neuropathol Appl Neurobiol. 1997;23:406–415. - PubMed
- Bansal R, Pfeiffer SE. FGF-2 converts mature oligodendrocytes to a novel phenotype. J Neurosci Res. 1997;50:215–228. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous