Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS - PubMed (original) (raw)
Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS
T D Palmer et al. J Neurosci. 1999.
Abstract
During development of the mammalian brain, both neurons and glia are generated from multipotent neural stem cells. Although neurogenesis ceases in most areas at birth, stem cells continue to generate neurons within the subventricular zone and hippocampal dentate gyrus throughout adult life. In this work, we provide the first demonstration that precursors native to regions of the adult brain that generate only glia can also generate neurons after exposure to FGF-2 in vitro. When progenitors isolated from hippocampal tissue were directly compared with cells isolated from the neocortex, both populations were able to initiate a program of proliferative neurogenesis. Genetic marking and lineage analysis showed that a majority of the cells able to generate neurons were multipotent precursors; however, progeny from these precursors acquired the competence to differentiate into neurons only after exposure to FGF-2. The recruitment of similar FGF-2-responsive cells from the adult optic nerve, a structure well isolated from the neurogenic zones within the brain, confirmed that neuron-competent precursors naturally exist in widely divergent tissues of the adult brain.
Figures
Fig. 1.
Isolation of endogenously proliferating cells from the adult hippocampus and cortex. A, Coronal section of the cortex and hippocampus of an adult rat immunostained for BrdU-labeled nuclei (white spots). Animals were injected with BrdU once each day for 6 d and then evaluated on the seventh day. Proliferative cells were abundant within the subgranule zone of the hippocampal dentate gyrus (b, B) and fimbrial ridge of the hippocampus (d, D). Closely juxtaposed pairs of daughter cells are clearly seen in white matter tracts, suggesting division in situ(D, E). Acid-stable vimentin immunoreactivity (blue) is clearly shown in the specialized ependymal cells lining the fimbrial surface. Although BrdU-positive proliferative cells were present in the cortical gray matter (c, C), they were much more abundant in subcortical white matter and adjacent ventricular zones (e, E). F, Tissues used for cultures included whole hippocampal lobes, cortical ribbons lateral to the midline (approximate lateral boundaries show by white bars in A), and sections of optic nerve rostral to the optic chiasma. G, Tissue was enzymatically dissociated and fractionated over a 50% Percoll gradient. Colored beads of known buoyancy were used to calibrate the gradient (left). The specific gravity of beads flanking the separation zone is shown (in grams per milliliter). Fractionated tissues generated two major bands of nucleated cells (right). The top band contained differentiated cells, myelin, and tissue debris. The bottom band contained undifferentiated progenitors and ependymal cells. A band of RBCs also formed immediately below the progenitor population (not visible here). H, The migration of progenitors in the gradient relative to that of calibrated beads demonstrates that adult-derived neural progenitors have a relatively high-buoyancy density of 1.065–1.075 gm/ml.
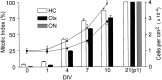
Fig. 2.
Proliferation of progenitors after exposure to FGF-2. Cells from the low-buoyancy fraction were plated at a density of 104/cm2 into defined medium containing 20 ng/ml FGF-2. To monitor proliferation, cells were pulsed with BrdU for 24 hr before being fixed after the indicated number of DIV, with the exception of day 21 in which each culture was passaged once and the cells labeled with BrdU for 72 hr before being fixed. At day 21, cultures initiated from optic nerve were also included, and >50,000 cells were scored in each culture. All cells were labeled, demonstrating that all cells were proliferative. The total number of cells per square centimeter was determined by counting nuclei (lines), whereas the percent of cells labeled with BrdU (mitotic index) was determined using immunofluorescent staining (bars). Values are mean ± SEM;n = 3 independent isolates. Ctx, Cortex; ON, optic nerve.
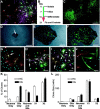
Fig. 3.
Lineage potential of freshly isolated progenitors. To determine the lineage potential of low-buoyancy cells from cortex or hippocampus, cells were cultured for 14 d and then allowed to differentiate (cortical cells shown in A–J).A, Bulk populations contained all three neural lineages. β-Tubulin-positive neurons are shown in red, GFAP-positive astrocytes in green, immature O4-positive oligodendrocytes in magenta, and nuclei in_blue_. B, To evaluate the lineage potential of single cells, cultures were treated with retroviral vectors on day 7, allowed to proliferate, and then induced to differentiate. Cell phenotypes were evaluated on day 21.C, A large variability in clone sizes and morphologies can be seen in the GFP-positive clones generated from an excess of virus. One hundred-fold less virus was used to generate well-separated clones scored in D–L.D–F, The locations of single green cells were marked 24–36 hr after infection (arrow in D). The same clone is shown at 5 d after infection and after fixation at day 21 (E, F).G–J, Lineage-specific markers were evaluated within each clone. Tubulin is shown in red, GFAP in_magenta_, and nuclei in blue.G, A two-cell clone containing only neurons. The neuronal cluster contains seven or more cells, but only two are marked with GFP (orange where red and_green_ overlap), suggesting that the virally marked cell was resident within a larger clone of neuroblasts.H, A clone containing only glia. Although neurons are present in this field (red), the GFP-marked clone (green) only contains GFAP-positive astrocytes (magenta within the green staining cell bodies, arrow). I, Both β-tubulin-positive (orange, arrow) and GFAP-positive cells (magenta and green_overlay, arrowhead) are present in this clone, demonstrating that the infected precursor was multipotent.J, Many small clones of large bipolar cells were negative for neuronal or glial markers. These cells grew slowly and were eventually outgrown by the neural progenitors in long-term cultures. K, L, Clones from at least three independent tissue preparations were scored for lineage and size (number of GFP-labeled cells per clone). Neurons Only, Only β-tubulin-positive cells; Glia Only, only GFAP- and/or O4-positive cells; Neurons and Glia, colonies with β-tubulin- and GFAP-positive cells; X1, large vimentin-positive clones negative for neuronal or glial markers similar to the large clone in the bottom left corner of_C; X2, small clones containing large bipolar cells as in J. Values are mean ± SEM. Scale bars: A, E–J, 50 μm;C, D, 400 μm.
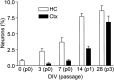
Fig. 4.
Exposure to FGF-2 activates a neurogenic potential in progenitors from cortex. Cells from the hippocampus or cortex were cultured for various times in FGF-2 and then allowed to differentiate for 7 d. Without previous exposure to FGF-2, cortex-derived cells were unable to generate β-tubulin-positive neurons (<0.001% at day 0). With as little as 3 d of FGF-2 exposure, some cortical progenitors began to generate neurons and, with increasing passage, cortical and hippocampal cultures normalized to a steady-state population, ∼10% of which were capable of differentiating into neurons.
Fig. 5.
Neurogenesis in cultures initiated from adult optic nerve. Low-buoyancy cells were isolated from adult optic nerve and placed in culture. At 1 DIV, a mixture of immature and differentiated cell morphologies was present (A).Arrows in A show typical glial profiles present at 1 DIV. On days 3 (B) and 7 (C), small proliferating clusters became apparent. After 3 weeks in the presence of FGF-2, a small but significant proportion of cells (0.8%) were able to differentiate into β-tubulin-positive neurons. In D–K, cells were treated with BrdU for 72 hr (days 12–14) and then switched to differentiation medium on day 14. Cells were fixed on day 28 and evaluated for BrdU and lineage-specific markers. Total nuclei are shown in blue (D, G). BrdU-positive nuclei (white) in the same fields are shown along with β-tubulin (red, E) or 200 kDa neurofilament (green, H).F and I, respectively, show multiple labeling for β-tubulin (red), GFAP (green), and O4 (magenta) or 200 kDa neurofilament (green), tau (red), and GFAP (magenta). Enlargements of the areas boxed in E and_H_ clearly show that the neuronal nuclei are BrdU-positive (white in J and_K_). Scale bars: A–C, 60 μm;D–I, 35 μm; J, K, 15 μm.
Fig. 6.
Potential influence of FGF-2 on the adult neural stem cell. If multipotent precursors or stem cells (SC) exist in non-neurogenic areas of the brain, they may persist in one of two states. Multipotent cells may actively participate in the gliogenic program by replenishing a pool of committed progenitors (P). Removal of cells from this environment and treatment with FGF-2 may de-repress or stimulate a latent neurogenic program. Alternatively, stem cells may represent a vestigial population that is inactive in most areas of the brain. Native gliogenesis (and/or neurogenesis) would then be mediated by committed progenitors, and the primary effects of FGF-2 on stem cells isolated in culture would be mitogenic. Once actively dividing in culture, stem cells may generate glia (G) or neurons (N) in a stochastic manner.
Similar articles
- Isolation of multipotent neural precursors residing in the cortex of the adult human brain.
Arsenijevic Y, Villemure JG, Brunet JF, Bloch JJ, Déglon N, Kostic C, Zurn A, Aebischer P. Arsenijevic Y, et al. Exp Neurol. 2001 Jul;170(1):48-62. doi: 10.1006/exnr.2001.7691. Exp Neurol. 2001. PMID: 11421583 - Developmental expression of fibroblast growth factor (FGF) receptors in neural stem cell progeny. Modulation of neuronal and glial lineages by basic FGF treatment.
Reimers D, López-Toledano MA, Mason I, Cuevas P, Redondo C, Herranz AS, Lobo MV, Bazán E. Reimers D, et al. Neurol Res. 2001 Sep;23(6):612-21. doi: 10.1179/016164101101199090. Neurol Res. 2001. PMID: 11547930 - FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain.
Palmer TD, Ray J, Gage FH. Palmer TD, et al. Mol Cell Neurosci. 1995 Oct;6(5):474-86. doi: 10.1006/mcne.1995.1035. Mol Cell Neurosci. 1995. PMID: 8581317 - Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells.
Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Emsley JG, et al. Prog Neurobiol. 2005 Apr;75(5):321-41. doi: 10.1016/j.pneurobio.2005.04.002. Prog Neurobiol. 2005. PMID: 15913880 Review. - Postnatal cerebral cortical multipotent progenitors: regulatory mechanisms and potential role in the development of novel neural regenerative strategies.
Mehler MF, Gokhan S. Mehler MF, et al. Brain Pathol. 1999 Jul;9(3):515-26. doi: 10.1111/j.1750-3639.1999.tb00539.x. Brain Pathol. 1999. PMID: 10416991 Free PMC article. Review.
Cited by
- Fractone-heparan sulphates mediate FGF-2 stimulation of cell proliferation in the adult subventricular zone.
Douet V, Kerever A, Arikawa-Hirasawa E, Mercier F. Douet V, et al. Cell Prolif. 2013 Apr;46(2):137-45. doi: 10.1111/cpr.12023. Cell Prolif. 2013. PMID: 23510468 Free PMC article. - The Mineralocorticoid Agonist Fludrocortisone Promotes Survival and Proliferation of Adult Hippocampal Progenitors.
Gesmundo I, Villanova T, Gargantini E, Arvat E, Ghigo E, Granata R. Gesmundo I, et al. Front Endocrinol (Lausanne). 2016 Jun 16;7:66. doi: 10.3389/fendo.2016.00066. eCollection 2016. Front Endocrinol (Lausanne). 2016. PMID: 27379018 Free PMC article. - Stem cells from birth to death: The history and the future.
de Haan G, Van Zant G. de Haan G, et al. J Am Aging Assoc. 2002 Apr;25(2):79-86. doi: 10.1007/s11357-002-0006-z. J Am Aging Assoc. 2002. PMID: 23604899 Free PMC article. - Prospects for neural stem cell-based therapies for neurological diseases.
Imitola J. Imitola J. Neurotherapeutics. 2007 Oct;4(4):701-14. doi: 10.1016/j.nurt.2007.08.005. Neurotherapeutics. 2007. PMID: 17920551 Free PMC article. Review. - Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents.
Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Gritti A, et al. J Neurosci. 2002 Jan 15;22(2):437-45. doi: 10.1523/JNEUROSCI.22-02-00437.2002. J Neurosci. 2002. PMID: 11784788 Free PMC article.
References
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. - PubMed
- Altman J, Das GD. Autoradiographic and histological studies of postnatal neurogenesis. I. A longitudinal investigation of the kinetics, migration and transformation of cells incorporating tritiated thymidine in neonate rats, with special reference to postnatal neurogenesis in some brain regions. J Comp Neurol. 1966;126:337–389. - PubMed
- Anderson DJ. Cell fate determination in the peripheral nervous system: the sympathoadrenal progenitor. J Neurobiol. 1993;24:185–198. - PubMed
- Anderson DJ. Stem cells and transcription factors in the development of the mammalian neural crest. FASEB J. 1994;8:707–713. - PubMed
- Aparicio OM, Gottschling DE. Overcoming telomeric silencing: a trans-activator competes to establish gene expression in a cell cycle-dependent way. Genes Dev. 1994;8:1133–1146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical