Analysis of F factor TraD membrane topology by use of gene fusions and trypsin-sensitive insertions - PubMed (original) (raw)
Analysis of F factor TraD membrane topology by use of gene fusions and trypsin-sensitive insertions
M H Lee et al. J Bacteriol. 1999 Oct.
Abstract
This report describes a procedure for characterizing membrane protein topology which combines the analysis of reporter protein hybrids and trypsin-sensitive 31-amino-acid insertions generated by using transposons ISphoA/in and ISlacZ/in. Studies of the F factor TraD protein imply that the protein takes on a structure with two membrane-spanning sequences and amino and carboxyl termini facing the cytoplasm. It was possible to assign the subcellular location of one region for which the behavior of fused reporter proteins was ambiguous, based on the trypsin cleavage behavior of a 31-residue insertion.
Figures
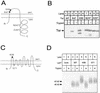
FIG. 1
Trypsin cleavage of insertion derivatives of lac permease and Tsr. (A) Positions in the Tsr topology of the 31-residue insertions examined. Closed circles denote trypsin sensitivity in spheroplasts, and open circles denote trypsin resistance. The alkaline phosphatase activities of cells expressing the tsr_-IS_phoA/in insertions used to generate the 31-residue insertions indicated were measured for plasmids in strain CC118. The alkaline phosphatase activities (expressed in activity units ± standard deviation) were as follows: no plasmid, 1.7 ± 0.1; R47, 2162 ± 49; E89, 2589 ± 22; N247, 14 ± 1; and A397, 8 ± 0.4. peri., periplasmic; cyto., cytoplasmic. (B) Western blot of Tsr insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against the inserted sequence was used to develop the blot (25). The trypsin treatment led to the production of polypeptides approximately the sizes expected for N-terminal fragments resulting from cleavage within the two periplasmic inserts (∼6.5 kDa for R47 and ∼15 kDa for E89) (not shown). Trypsin treatment procedure 2 with cells grown at 30°C was used. (C) Positions in the lac permease topology of the N38 and G71 31-residue insertions. The closed circle indicates trypsin sensitivity in spheroplasts; the open circle indicates resistance. (D) Western blot of lac permease insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against the C terminus of lactose permease was used to develop the blot. lac permease runs as a broad band in SDS-PAGE. Strain CC874 carrying different plasmids was analyzed by trypsin treatment procedure 1 with cells grown at 37°C. WT, wild type.
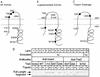
FIG. 2
Analysis of TraD topology. (A) Locations in the favored two-span model and alkaline phosphatase activities of nine TraD-alkaline phosphatase hybrids. Closed circles denote high activity (greater than 150 Miller units), open circles denote low activity (less than 25 Miller units), and the gray circle denotes an intermediate value (46 Miller units) (Table 2). The thick gray line denotes the location of a potential third membrane-spanning sequence. (B) Locations and β-galactosidase activities of eight TraD–β-galactosidase hybrids. Closed circles denote high activity (I14, 183 U, K252, 133 U; V343, 145 U; N702, 127 U), open circles denote low activity (I59, 2 U; T65, 6 U), and the gray circles denote intermediate activity (Q94, 48 U; E106, 70 U). (C) Positions in the topology and cleavage by trypsin in spheroplasts of four 31-amino-acid insertions in TraD. Closed circles represent insertions that were trypsin sensitive; open circles represent trypsin-resistant insertions. (D) Western blot of TraD insertion derivatives following trypsin treatment of spheroplasts. A polyclonal antiserum directed against either the inserted sequence or wild-type TraD was used as indicated previously (25). A strain deleted for traD was used as a negative control (none). A strain expressing wild-type TraD (WT) served as a negative control in the antiinsert blot. An aliquot of the spheroplast preparation expressing the N702 insertion derivative was sonicated before the addition of trypsin (lanes 17 and 18). Trypsin treatment procedure 1 was used with cells grown at 37°C.
Similar articles
- Use of phoA and lacZ fusions to study the membrane topology of ProW, a component of the osmoregulated ProU transport system of Escherichia coli.
Haardt M, Bremer E. Haardt M, et al. J Bacteriol. 1996 Sep;178(18):5370-81. doi: 10.1128/jb.178.18.5370-5381.1996. J Bacteriol. 1996. PMID: 8808924 Free PMC article. - Membrane topology of the Rickettsia prowazekii ATP/ADP translocase revealed by novel dual pho-lac reporters.
Alexeyev MF, Winkler HH. Alexeyev MF, et al. J Mol Biol. 1999 Jan 29;285(4):1503-13. doi: 10.1006/jmbi.1998.2412. J Mol Biol. 1999. PMID: 9917392 - Analysis of the topology of a membrane protein by using a minimum number of alkaline phosphatase fusions.
Boyd D, Traxler B, Beckwith J. Boyd D, et al. J Bacteriol. 1993 Jan;175(2):553-6. doi: 10.1128/jb.175.2.553-556.1993. J Bacteriol. 1993. PMID: 8419303 Free PMC article. - Gene fusion approaches to membrane protein topology.
Boyd D, Traxler B, Jander G, Prinz W, Beckwith J. Boyd D, et al. Soc Gen Physiol Ser. 1993;48:23-37. Soc Gen Physiol Ser. 1993. PMID: 8503047 Review. No abstract available. - Analysis of membrane protein topology using alkaline phosphatase and beta-galactosidase gene fusions.
Manoil C. Manoil C. Methods Cell Biol. 1991;34:61-75. doi: 10.1016/s0091-679x(08)61676-3. Methods Cell Biol. 1991. PMID: 1943817 Review. No abstract available.
Cited by
- Analysis of functional domains of the Enterococcus faecalis pheromone-induced surface protein aggregation substance.
Waters CM, Dunny GM. Waters CM, et al. J Bacteriol. 2001 Oct;183(19):5659-67. doi: 10.1128/JB.183.19.5659-5667.2001. J Bacteriol. 2001. PMID: 11544229 Free PMC article. - Plasmid r1 conjugative DNA processing is regulated at the coupling protein interface.
Mihajlovic S, Lang S, Sut MV, Strohmaier H, Gruber CJ, Koraimann G, Cabezón E, Moncalián G, de la Cruz F, Zechner EL. Mihajlovic S, et al. J Bacteriol. 2009 Nov;191(22):6877-87. doi: 10.1128/JB.00918-09. Epub 2009 Sep 18. J Bacteriol. 2009. PMID: 19767437 Free PMC article. - Rational engineering of a virulence gene from Mycobacterium tuberculosis facilitates proteomic analysis of a natural protein N-terminus.
Reyna C, Mba Medie F, Champion MM, Champion PA. Reyna C, et al. Sci Rep. 2016 Sep 14;6:33265. doi: 10.1038/srep33265. Sci Rep. 2016. PMID: 27625110 Free PMC article. - General mutagenesis of F plasmid TraI reveals its role in conjugative regulation.
Haft RJ, Palacios G, Nguyen T, Mally M, Gachelet EG, Zechner EL, Traxler B. Haft RJ, et al. J Bacteriol. 2006 Sep;188(17):6346-53. doi: 10.1128/JB.00462-06. J Bacteriol. 2006. PMID: 16923902 Free PMC article. - Biogenesis, architecture, and function of bacterial type IV secretion systems.
Christie PJ, Atmakuri K, Krishnamoorthy V, Jakubowski S, Cascales E. Christie PJ, et al. Annu Rev Microbiol. 2005;59:451-85. doi: 10.1146/annurev.micro.58.030603.123630. Annu Rev Microbiol. 2005. PMID: 16153176 Free PMC article. Review.
References
- Calamia J, Manoil C. Membrane protein spanning segments as export signals. J Mol Biol. 1992;224:539–543. - PubMed
- Das A, Xie Y-H. Construction of transposon Tn3phoA: its application in defining the membrane topology of the Agrobacterium tumefaciens DNA transfer proteins. Mol Microbiol. 1998;27:405–414. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources