Enhancement by galactosamine of lipopolysaccharide(LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment - PubMed (original) (raw)
Enhancement by galactosamine of lipopolysaccharide(LPS)-induced tumour necrosis factor production and lethality: its suppression by LPS pretreatment
Y Endo et al. Br J Pharmacol. 1999 Sep.
Abstract
1. D-Galactosamine (GalN) depletes UTP primarily in the liver, resulting in decreased RNA synthesis in hepatocytes. Co-injection of GalN and lipopolysaccharide (LPS) into mice produces fulminant hepatitis with severe hepatic congestion, resulting in rapid death. Although the underlying mechanism is uncertain, GalN enhances the sensitivity to tumour necrosis factor (TNF). Administration of uridine (a precursor of UTP) prior injection of either LPS itself or interleukin-1 (IL-1) reduces the lethality of GalN+LPS. The present study focused on the effects of these agents on TNF production. 2. Intraperitoneal injection of GalN+LPS into mice greatly elevated serum TNF. Although large doses of LPS alone also greatly elevated serum TNF, LPS itself induced neither hepatic congestion nor rapid death. Administration of a macrophage depletor, liposomes encapsulated with dichloromethylene bisphosphonate, reduced both the TNF production and mortality induced by GalN+LPS. 3. Uridine, when injected 0.5 h after the injection of GalN+LPS, reduced the production of TNF. Prior injection of LPS, but not of IL-1, also reduced this TNF production. 4. Serum from LPS-injected mice reduced the TNF production induced by GalN+LPS, but it was less effective at reducing the lethality. Its ability to reduce TNF production was abolished by heat-treatment. 5. We hypothesize that a factor inhibiting TNF production by macrophages is produced by hepatocytes in response to LPS. Possibly, production of this hepatocyte-derived TNF-down-regulator (TNF-DRh) may be: (i) inhibited by GalN, causing over-production of TNF by macrophages and (ii) stimulated by LPS-pretreatment (and restored by uridine), causing reduced TNF production.
Figures
Figure 1
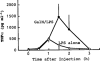
Elevation of serum TNFα, IL-1α and IL-1β following injection of LPS alone. Mice were killed at the indicated times after the injection of LPS (10 μg kg−1, i.p.). Values are mean±s.d. from four mice.
Figure 2
Elevation of serum TNFα following injection of LPS plus GalN. Mice were killed at the indicated times after the co-injection of LPS (10 μg kg−1, i.p.) and GalN (600 mg kg−1, i.p.). The effect on TNFα levels induced by LPS alone (shown in Figure 1) is also shown in this Figure for comparison. Values are mean±s.d. from four mice.
Figure 3
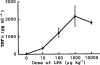
Dose-dependency of the TNFα production induced by LPS alone. The mice were killed 1.5 h after the i.p. injection of various doses of LPS and blood was then collected. Values are mean±s.d. from four mice.
Figure 4
Effect of uridine on the TNFα production induced by co-injection of LPS and GalN. Mice were given a 1st injection (i.p.) of saline (S), LPS (10 μg kg−1), GalN (600 mg kg−1) or LPS plus GalN (10 μg kg−1 and 600 mg kg−1, respectively). Then, 0.5 h later they were given a 2nd injection (i.p.) consisting of either saline or uridine (Ur) (500 mg kg−1). The mice were killed 1.5 h after the 2nd injection and blood was then collected. Values are mean±s.d. from four mice. *P<0.01 vs S→S, #P<0.01 vs LPS→S, †P<0.05 vs LPS/GalN→S.
Figure 5
Effects of LPS-pretreatment on the TNFα and IL-1α production induced by GalN+LPS. Mice were given (i.p.) saline (S) or LPS (10 μg kg−1), then 3 h later they were given a 2nd i.p. injection consisting of saline, LPS (10 μg kg−1) or LPS plus GalN (10 μg kg−1 and 600 mg kg−1, respectively). The mice were killed 1.5 h after the 2nd injection and blood was then collected. Values are mean±s.d. from four mice. *P<0.01 vs S→S, #P<0.01 vs S→LPS, †P<0.05 vs S→LPS, ‡P<0.01 vs S→LPS/GalN.
Figure 6
Effects of pretreatment with IL-1 or Cl2MBP-liposomes on the TNFα production induced by GalN+LPS. Mice were given (i.p.) saline (S) or IL-1 (5 μg kg−1) then 3 h later they were given a 2nd i.p. injection consisting of either saline or GalN+LPS (600 mg and 10 μg kg−1, respectively). The mice were killed 1.5 h after the 2nd injection and blood was then collected. In some mice, an injection of Cl2MBP-liposomes (Lip) (original suspension) was given i.v. 24 h before the injection of GalN+LPS. Values are mean±s.d. from four mice. *P<0.01 vs S→GalN/LPS.
Figure 7
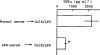
Effect of serum from mice given LPS on the TNFα production induced by GalN+LPS. Serum (0.1 ml per mouse) taken from normal mice or from mice injected with LPS (0.5 mg kg−1, i.p.) 24 h previously (LPS-serum) was injected (i.v.) into other mice, and 10 min later GalN+LPS was injected. The mice were killed 1.5 h after the second injection and blood was then collected. Values are mean±s.d. from four mice. *P<0.01 vs normal serum→GalN/LPS.
Figure 8
Effect of serum from mice given LPS on the TNFα production induced by LPS alone. Serum taken from normal mice (normal serum) was used without dilution, while serum taken from mice injected with LPS (0.5 mg kg−1, i.p.) 24 h previously (LPS-serum) was diluted with saline as indicated in parenthesis. The normal serum, diluted LPS-serum or saline was injected into other mice (0.1 ml/mouse, i.v.) and, 10 min later, LPS was injected (0.1 mg kg−1, i.v.). The mice were killed 1.5 h after the second injection and blood was then collected. In some experiments (indicated by the superscript a), the LPS-serum diluted to 1/3 was exposed to boiling water for 10 min and then cooled to 37°C in water. Values are mean±s.d. from four mice. *P<0.01 vs normal serum→LPS.
Similar articles
- Ornithine and histidine decarboxylase activities in mice sensitized to endotoxin, interleukin-1 or tumour necrosis factor by D-galactosamine.
Endo Y, Kikuchi T, Nakamura M. Endo Y, et al. Br J Pharmacol. 1992 Nov;107(3):888-94. doi: 10.1111/j.1476-5381.1992.tb14542.x. Br J Pharmacol. 1992. PMID: 1472981 Free PMC article. - Secoisolariciresinol and isotaxiresinol inhibit tumor necrosis factor-alpha-dependent hepatic apoptosis in mice.
Banskota AH, Nguyen NT, Tezuka Y, Le Tran Q, Nobukawa T, Kurashige Y, Sasahara M, Kadota S. Banskota AH, et al. Life Sci. 2004 Apr 16;74(22):2781-92. doi: 10.1016/j.lfs.2003.10.021. Life Sci. 2004. PMID: 15043992 - Protective effect of SKLB010 against D-galactosamine/lipopolysaccharide-induced acute liver failure via nuclear factor-κB signaling pathway in macrophages.
Xie C, Jingjing W, Li X, Zeng F, Ma L, Li C, Wei Z, Peng A, Chen L. Xie C, et al. Int Immunopharmacol. 2014 Aug;21(2):261-8. doi: 10.1016/j.intimp.2014.05.012. Epub 2014 May 24. Int Immunopharmacol. 2014. PMID: 24861250 - Natural production and release of tumour necrosis factor.
Gifford GE, Flick DA. Gifford GE, et al. Ciba Found Symp. 1987;131:3-20. doi: 10.1002/9780470513521.ch2. Ciba Found Symp. 1987. PMID: 3131075 Review.
Cited by
- The Protective Effect of Low Dose of Lipopolysaccharide Pretreatment on Endotoxin-Induced Uveitis in Rats Is Associated with Downregulation of CSF-1 and Upregulation of LRR-1.
Ling Y, Wang J, Yu S, Li W, Lu H. Ling Y, et al. J Immunol Res. 2020 Jul 1;2020:9314756. doi: 10.1155/2020/9314756. eCollection 2020. J Immunol Res. 2020. PMID: 32671118 Free PMC article. - Scavenger receptor A-mediated nanoparticles target M1 macrophages for acute liver injury.
Zhang R, Luo S, Zhao T, Wu M, Huang L, Zhang L, Huang Y, Gao H, Sun X, Gong T, Zhang Z. Zhang R, et al. Asian J Pharm Sci. 2023 May;18(3):100813. doi: 10.1016/j.ajps.2023.100813. Epub 2023 May 9. Asian J Pharm Sci. 2023. PMID: 37274920 Free PMC article. - Asiatic Acid Exhibits Anti-inflammatory and Antioxidant Activities against Lipopolysaccharide and d-Galactosamine-Induced Fulminant Hepatic Failure.
Lv H, Qi Z, Wang S, Feng H, Deng X, Ci X. Lv H, et al. Front Immunol. 2017 Jul 7;8:785. doi: 10.3389/fimmu.2017.00785. eCollection 2017. Front Immunol. 2017. PMID: 28736552 Free PMC article. - Low Dose of Lipopolysaccharide Pretreatment Preventing Subsequent Endotoxin-Induced Uveitis Is Associated with PI3K/AKT Pathway.
Zhang N, Yu S, Liu X, Lu H. Zhang N, et al. J Immunol Res. 2017;2017:1273940. doi: 10.1155/2017/1273940. Epub 2017 Jul 18. J Immunol Res. 2017. PMID: 28804726 Free PMC article. - Platycodon grandiflorus Fermented Extracts Attenuate Endotoxin-Induced Acute Liver Injury in Mice.
Kim SR, Park EJ, Dusabimana T, Je J, Jeong K, Yun SP, Kim HJ, Cho KM, Kim H, Park SW. Kim SR, et al. Nutrients. 2020 Sep 13;12(9):2802. doi: 10.3390/nu12092802. Nutrients. 2020. PMID: 32933130 Free PMC article.
References
- AUSTYN J.M., GORDON S. F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur. J. Immunol. 1981;11:805–815. - PubMed
- CZAJA M.J., SCHILSKY M.L., XU Y., SCHMIEDEBERG P., COMPTON A., RIDNOUR L., OBERLEY L.W. Induction of MnSOD gene expression in a hepatic model of TNF-α toxicity does not result in increased protein. Am. J. Physiol. 1994;266:G737–G744. - PubMed
- DECKER K., KEPPLER D. Galactosamine hepatitis: key role of the nucleotide deficiency period in the pathogenesis of cell injury and cell death. Rev. Physiol. Biochem. Pharmacol. 1974;71:77–106. - PubMed
- DESIDERIO M.A., GRASSILLI E., BELLESIA E., SALOMONI P., FRANCESCHI C. Involvement of ornithine decarboxylase and polyamines in glucocorticoid-induced apoptosis of rat thymocytes. Cell Growth Differ. 1995;6:505–513. - PubMed
- DINARELLO C.A. Interleukin-1 and its biologically related cytokines. Adv. Immunol. 1989;44:153–205. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical