Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element - PubMed (original) (raw)
Estrogen induction of the cyclin D1 promoter: involvement of a cAMP response-like element
M Sabbah et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Estrogens induce cell proliferation in target tissues by stimulating progression through the G(1) phase of the cell cycle. Induction of cyclin D1 expression is a critical feature of the mitogenic action of estrogen. We have determined a region between -96 and -29 in the cyclin D1 promoter that confers regulation by estrogens in the human mammary carcinoma cells MCF-7. This region encompasses a unique known transcription factor binding site with a sequence of a potential cAMP response element (CRE-D1). The induction is strictly hormone dependent and requires the DNA binding domain as well as both AF-1 and AF-2 domains of the estrogen receptor (ER) alpha. Destruction of the CRE-D1 motif caused complete loss of estrogen responsiveness. Both c-Jun and ATF-2 transactivated the cyclin D1 promoter in transient transfection experiments, and a clear additional increase was detected when ER was cotransfected with either c-Jun or with c-Jun and ATF-2 but not with ATF-2 alone. Furthermore, the expression of a dominant negative variant of c-Jun, TAM67, completely abolished the induction of the cyclin D1 promoter both in the absence and presence of ER. We show that ATF-2 homodimers and ATF-2/c-Jun heterodimers, but not c-Jun homodimers, were able to bind the CRE of the cyclin D1 promoter. To interpret these results, we propose a mechanism in which ATF-2/c-Jun heterodimers bind to the CRE-D1 element and mediate the activation of cyclin D1 promoter by the ER. This mechanism represents a pathway by which estrogens control the proliferation of target cells.
Figures
Figure 1
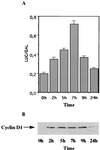
Transient activation of the cyclin D1 promoter by estradiol in MCF-7 cells. (A) MCF-7 cells were transfected by using the calcium phosphate procedure with 5 μg of D1–944 Luc and 1 μg of pCH110, then treated with 5 nM ICI 182,780 for 48 h in the presence of 5% charcoal-stripped serum (CSS). At t = 0 h, medium was changed to 5% CSS with 10 nM estradiol. At the times indicated, cells were harvested and assayed for luciferase and β-galactosidase activities. (B) Whole-cell extracts were prepared from a parallel set of transfected cells and analyzed for cyclin D1 protein by Western blotting.
Figure 2
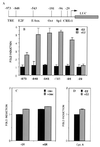
The −96-bp region of the cyclin D1 promoter is required for activation by ER. (A) Schematic representation of the intact and 5′ deleted human cyclin D1 promoter luciferase constructs. Numbers at the top indicate the endpoints of the 5′ deletion mutants starting from the Inr element. The 3′ end of the D1 promoter in the reporter constructs is +139. Positions of the known regulatory elements are shown. HeLa cells were transfected by using Lipofectamine with: (B) 1 μg of luciferase reporter gene driven by 5′ deletion mutants of the human cyclin D1 promoter, 0.2 μg of pSG5-ER, and 0.2 μg of pCH110 as an internal standard of transfection efficiency. After 24 h, cells were incubated with 10 nM estradiol or the ethanol vehicle for 24 h. (C) 1 μg of D1–96 Luc, 0.2 μg of pSG5-GR, and 0.2 μg of pCH110. After 24 h, cells were incubated with 100 nM dexamethasone or ethanol vehicle for 24 h. (D) 1 μg of luciferase reporter gene driven by a 500-bp fragment of the human cyclin A promoter, 0.2 μg of pSG5-ER, and 0.2 μg of pCH110. After 24 h, cells were incubated with 10 nM estradiol or the ethanol vehicle for 24 h. At 48 h posttransfection, cells were harvested and luciferase activities were measured and normalized to β-galactosidase activities.
Figure 3
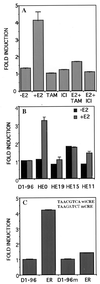
Induction of the D1–96 promoter by estradiol requires the presence of wild-type ER and integrity of the CRE-binding site. (A) HeLa cells were transfected with D1–96 Luc, pSG5-ER, and pCH110 as described in Fig. 2_B_. After 24 h, cells were incubated with 10 nM estradiol and/or 100 nM tamoxifen, and/or 5 nM ICI 182,780 or ethanol vehicle for 24 h. (B) Deletion mutants of the ER were transfected with D1–96 Luc and pCH110. After 24 h cells were incubated with 10 nM estradiol or ethanol vehicle for 24 h. (C) Effect of CRE mutation (Inset) on the activation of the D1–96 promoter by ER. The pSG5-ER was cotransfected with D1–96 Luc or with D1–96m Luc and pCH110 in HeLa cells. After 24 h, cells were incubated with 10 nM estradiol for 24 h. At 48 h posttransfection cells were harvested and luciferase activities were measured and normalized to β-galactosidase activities.
Figure 4
ER requires c-Jun to mediate cyclin D1 promoter activation. HeLa cells were transfected as described in Fig. 2_B_ with: (A) D1–96 Luc together with expression vectors for ER, c-Jun, c-Fos, and ATF-2 alone or in combinations as indicated. (B) D1–96 Luc and ER and/or TAM 67. After 24 h, cells were incubated with 10 nM estradiol for 24 h. At the end of incubation cells were harvested and luciferase activities were measured and normalized to β-galactosidase activities. F, c-Fos; J, c-Jun; A, ATF-2. C. In vitro interaction of c-Jun and ATF-2 with ER. 35S-labeled c-Jun and ATF-2 were prepared by translation in vitro. Labeled protein probes were incubated with immobilized GST or GST-ER mutants. Equal amounts of 35S-labeled proteins were incubated with each resin. After extensive washing of the GST beads, the proteins were eluted, separated on PAGE, and detected by fluorography. The input lanes contained 10% of the labeled proteins.
Figure 5
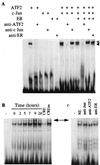
c-Jun and ATF-2 proteins specifically bind to the cyclin D1-CRE. (A) The 32P-labeled DNA probe (≈25,000 cpm) was incubated for 30 min at room temperature with the in vitro_-translated c-Jun, ATF-2 and ER proteins as indicated above each lane. For supershift assays, lysates were preincubated with the antisera for 15 min at room temperature before the addition of the radioactive probe. Open arrow indicates the ATF-2 homodimeric complexes and the closed arrow the ATF-2/c-Jun heterodimeric complexes. (B) Nuclear extracts (5 μg) of MCF-7 cells treated as described in Fig. 1_A were incubated with 2 μg of poly dI-dC for 15 min at 4°C before the addition of the radioactive cyclin D1-CRE probe. For competition experiments, a 100-fold molar excess of unlabeled CRE or mutated CRE was mixed with the radioactive probe. (C) Nuclear extracts (5 μg) of MCF-7 cells treated with estradiol for 7 h were preincubated with 2 μg of poly dI-dC for 15 min at 4°C, then antisera were added for 15 min at room temperature and finally with radioactive probe. The arrow indicates the position of c-Jun/ATF-2-DNA complexes.
Figure 6
Estrogens induce c-Jun expression. Nuclear extracts (30 μg) from MCF-7 cells treated as described in Fig. 1_A_ were analyzed for c-Jun and ATF-2 proteins by Western blotting. c-Jun-P indicates the phosphorylated form of c-Jun.
Similar articles
- pp60(v-src) induction of cyclin D1 requires collaborative interactions between the extracellular signal-regulated kinase, p38, and Jun kinase pathways. A role for cAMP response element-binding protein and activating transcription factor-2 in pp60(v-src) signaling in breast cancer cells.
Lee RJ, Albanese C, Stenger RJ, Watanabe G, Inghirami G, Haines GK 3rd, Webster M, Muller WJ, Brugge JS, Davis RJ, Pestell RG. Lee RJ, et al. J Biol Chem. 1999 Mar 12;274(11):7341-50. doi: 10.1074/jbc.274.11.7341. J Biol Chem. 1999. PMID: 10066798 - Estrogens and progesterone promote persistent CCND1 gene activation during G1 by inducing transcriptional derepression via c-Jun/c-Fos/estrogen receptor (progesterone receptor) complex assembly to a distal regulatory element and recruitment of cyclin D1 to its own gene promoter.
Cicatiello L, Addeo R, Sasso A, Altucci L, Petrizzi VB, Borgo R, Cancemi M, Caporali S, Caristi S, Scafoglio C, Teti D, Bresciani F, Perillo B, Weisz A. Cicatiello L, et al. Mol Cell Biol. 2004 Aug;24(16):7260-74. doi: 10.1128/MCB.24.16.7260-7274.2004. Mol Cell Biol. 2004. PMID: 15282324 Free PMC article. - Activating transcription factor 2 targets c-Fos, but not c-Jun, in growth plate chondrocytes.
Li X, LuValle P. Li X, et al. J Cell Biochem. 2011 Jan;112(1):211-6. doi: 10.1002/jcb.22925. J Cell Biochem. 2011. PMID: 21069729 - Identification of the cyclin D1 gene as a target of activating transcription factor 2 in chondrocytes.
Beier F, Lee RJ, Taylor AC, Pestell RG, LuValle P. Beier F, et al. Proc Natl Acad Sci U S A. 1999 Feb 16;96(4):1433-8. doi: 10.1073/pnas.96.4.1433. Proc Natl Acad Sci U S A. 1999. PMID: 9990041 Free PMC article.
Cited by
- Steroid Hormone Receptors: Links With Cell Cycle Machinery and Breast Cancer Progression.
Saha S, Dey S, Nath S. Saha S, et al. Front Oncol. 2021 Mar 12;11:620214. doi: 10.3389/fonc.2021.620214. eCollection 2021. Front Oncol. 2021. PMID: 33777765 Free PMC article. Review. - SIRT1 is involved in adrenocortical cancer growth and motility.
Chimento A, De Luca A, Nocito MC, Sculco S, Avena P, La Padula D, Zavaglia L, Sirianni R, Casaburi I, Pezzi V. Chimento A, et al. J Cell Mol Med. 2021 Apr;25(8):3856-3869. doi: 10.1111/jcmm.16317. Epub 2021 Mar 2. J Cell Mol Med. 2021. PMID: 33650791 Free PMC article. - The nuclear receptor liver receptor homolog-1 is an estrogen receptor target gene.
Annicotte JS, Chavey C, Servant N, Teyssier J, Bardin A, Licznar A, Badia E, Pujol P, Vignon F, Maudelonde T, Lazennec G, Cavailles V, Fajas L. Annicotte JS, et al. Oncogene. 2005 Dec 8;24(55):8167-75. doi: 10.1038/sj.onc.1208950. Oncogene. 2005. PMID: 16091743 Free PMC article. - The discovery and mechanism of action of letrozole.
Bhatnagar AS. Bhatnagar AS. Breast Cancer Res Treat. 2007;105 Suppl 1(Suppl 1):7-17. doi: 10.1007/s10549-007-9696-3. Epub 2007 Oct 3. Breast Cancer Res Treat. 2007. PMID: 17912633 Free PMC article. Review. - Elacestrant (RAD1901) exhibits anti-tumor activity in multiple ER+ breast cancer models resistant to CDK4/6 inhibitors.
Patel HK, Tao N, Lee KM, Huerta M, Arlt H, Mullarkey T, Troy S, Arteaga CL, Bihani T. Patel HK, et al. Breast Cancer Res. 2019 Dec 18;21(1):146. doi: 10.1186/s13058-019-1230-0. Breast Cancer Res. 2019. PMID: 31852484 Free PMC article.
References
- Clarke R, Dickson R B, Lippman M E. Crit Rev Oncol Hematol. 1992;12:1–23. - PubMed
- Sutherland R L, Hall R E, Taylor I W. Cancer Res. 1983;43:3998–4006. - PubMed
- Wakeling A E, Dukes M, Bowler J. Cancer Res. 1991;51:3867–3873. - PubMed
- Sicinski P, Donaher J L, Parker S B, Li T, Fazeli A, Gardner H, Haslam S Z, Bronson R T, Elledge S J, Weinberg R A. Cell. 1995;82:621–630. - PubMed
- Altucci L, Addeo R, Cicatiello L, Dauvois S, Parker M G, Truss M, Beato M, Sica V, Bresciani F, Weiss A. Oncogene. 1996;12:2315–2324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous