Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation - PubMed (original) (raw)
Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation
B Roozendaal et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Previously, we reported that bilateral excitotoxic lesions of the basolateral nucleus of the amygdala (BLA) block the enhancing effects of posttraining systemic or intrahippocampal glucocorticoid administration on memory for inhibitory avoidance training. The present study further examined the basis of this permissive influence of the BLA on hippocampal memory functioning. Immediate posttraining unilateral infusions of the specific glucocorticoid receptor agonist RU 28362 (11beta,17beta-dihydroxy-6, 21-dimethyl-17alpha-pregna-4,6-trien-20-yn-3-one; 3.0, 10.0, or 30.0 ng in 0.5 microliter) administered into the dorsal hippocampus of male Sprague-Dawley rats induced dose-dependent enhancement of 48-h inhibitory avoidance retention. Infusions of the beta-adrenoceptor antagonist atenolol (0.5 microgram in 0.2 microliter) into the ipsilateral, but not the contralateral, BLA 10 min prior to training blocked the hippocampal glucocorticoid effects on memory consolidation. Infusions of the muscarinic cholinergic antagonist atropine (0.5 microgram in 0.2 microliter) into either the ipsilateral or contralateral BLA before training did not block the hippocampal glucocorticoid effects. These findings provide further evidence that beta-adrenergic activity in the BLA is essential in enabling glucocorticoid-induced modulation of memory consolidation and are consistent with the hypothesis that the BLA regulates the strength of memory consolidation in other brain structures. The ipsilateral nature of the BLA-hippocampus interaction indicates that BLA influences on hippocampal memory processes are mediated through neural pathways rather than by influences by means of the activation of peripheral stress responses.
Figures
Figure 1
Photomicrographs illustrating the location of microinjection needle tips within the dorsal hippocampus (A) and basolateral amygdala (B). CA, Ammon’s horn; CEA, central amygdala; DG, dentate gyrus; LA, lateral amygdala. (×25)
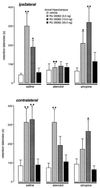
Figure 2
Retention latencies (mean ± SEM) in seconds of rats given immediate posttraining infusions of the GR agonist RU 28362 (3.0, 10.0, or 30.0 ng) into the dorsal hippocampus and pretraining infusions of either the β-adrenoceptor antagonist atenolol (0.5 μg in 0.2 μl) or the muscarinic cholinergic antagonist atropine (0.5 μg in 0.2 μl) into either the ipsilateral or contralateral basolateral amygdala. ∗, P < 0.05; ∗∗, P < 0.01, as compared with the corresponding intrahippocampal vehicle group; ♦♦, P < 0.01, as compared with the corresponding intra-BLA saline group. n = 8–12 animals per group.
Similar articles
- Glucocorticoid effects on memory retrieval require concurrent noradrenergic activity in the hippocampus and basolateral amygdala.
Roozendaal B, Hahn EL, Nathan SV, de Quervain DJ, McGaugh JL. Roozendaal B, et al. J Neurosci. 2004 Sep 15;24(37):8161-9. doi: 10.1523/JNEUROSCI.2574-04.2004. J Neurosci. 2004. PMID: 15371517 Free PMC article. - Glucocorticoid enhancement of memory storage involves noradrenergic activation in the basolateral amygdala.
Quirarte GL, Roozendaal B, McGaugh JL. Quirarte GL, et al. Proc Natl Acad Sci U S A. 1997 Dec 9;94(25):14048-53. doi: 10.1073/pnas.94.25.14048. Proc Natl Acad Sci U S A. 1997. PMID: 9391150 Free PMC article. - 1999 Curt P. Richter award. Glucocorticoids and the regulation of memory consolidation.
Roozendaal B. Roozendaal B. Psychoneuroendocrinology. 2000 Apr;25(3):213-38. doi: 10.1016/s0306-4530(99)00058-x. Psychoneuroendocrinology. 2000. PMID: 10737694 Review. - Memory consolidation and the amygdala: a systems perspective.
McGaugh JL. McGaugh JL. Trends Neurosci. 2002 Sep;25(9):456. doi: 10.1016/s0166-2236(02)02211-7. Trends Neurosci. 2002. PMID: 12183206 Review.
Cited by
- Molecular brake pad hypothesis: pulling off the brakes for emotional memory.
Vogel-Ciernia A, Wood MA. Vogel-Ciernia A, et al. Rev Neurosci. 2012;23(5-6):607-26. doi: 10.1515/revneuro-2012-0050. Rev Neurosci. 2012. PMID: 23096102 Free PMC article. Review. - Unique neural circuitry for neonatal olfactory learning.
Moriceau S, Sullivan RM. Moriceau S, et al. J Neurosci. 2004 Feb 4;24(5):1182-9. doi: 10.1523/JNEUROSCI.4578-03.2004. J Neurosci. 2004. PMID: 14762136 Free PMC article. - Involvement of the rostral anterior cingulate cortex in consolidation of inhibitory avoidance memory: interaction with the basolateral amygdala.
Malin EL, Ibrahim DY, Tu JW, McGaugh JL. Malin EL, et al. Neurobiol Learn Mem. 2007 Feb;87(2):295-302. doi: 10.1016/j.nlm.2006.09.004. Epub 2006 Oct 31. Neurobiol Learn Mem. 2007. PMID: 17079169 Free PMC article. - Glucocorticoid-induced enhancement of extinction-from animal models to clinical trials.
de Quervain D, Wolf OT, Roozendaal B. de Quervain D, et al. Psychopharmacology (Berl). 2019 Jan;236(1):183-199. doi: 10.1007/s00213-018-5116-0. Epub 2019 Jan 4. Psychopharmacology (Berl). 2019. PMID: 30610352 Free PMC article. Review. - Mechanisms of amygdala modulation of hippocampal plasticity.
Akirav I, Richter-Levin G. Akirav I, et al. J Neurosci. 2002 Nov 15;22(22):9912-21. doi: 10.1523/JNEUROSCI.22-22-09912.2002. J Neurosci. 2002. PMID: 12427848 Free PMC article.
References
- Cahill L, McGaugh J L. Trends Neurosci. 1998;17:208–214. - PubMed
- Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1997;67:176–179. - PubMed
- Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1996;65:1–8. - PubMed
- Roozendaal B, Portillo-Marquez G, McGaugh J L. Behav Neurosci. 1996;110:1074–1083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical