Aczonin, a 550-kD putative scaffolding protein of presynaptic active zones, shares homology regions with Rim and Bassoon and binds profilin - PubMed (original) (raw)
Aczonin, a 550-kD putative scaffolding protein of presynaptic active zones, shares homology regions with Rim and Bassoon and binds profilin
X Wang et al. J Cell Biol. 1999.
Abstract
Neurotransmitter exocytosis is restricted to the active zone, a specialized area of the presynaptic plasma membrane. We report the identification and initial characterization of aczonin, a neuron-specific 550-kD protein concentrated at the presynaptic active zone and associated with a detergent-resistant cytoskeletal subcellular fraction. Analysis of the amino acid sequences of chicken and mouse aczonin indicates an organization into multiple domains, including two pairs of Cys(4) zinc fingers, a polyproline tract, and a PDZ domain and two C2 domains near the COOH terminus. The second C2 domain is subject to differential splicing. Aczonin binds profilin, an actin-binding protein implicated in actin cytoskeletal dynamics. Large parts of aczonin, including the zinc finger, PDZ, and C2 domains, are homologous to Rim or to Bassoon, two other proteins concentrated in presynaptic active zones. We propose that aczonin is a scaffolding protein involved in the organization of the molecular architecture of synaptic active zones and in the orchestration of neurotransmitter vesicle trafficking.
Figures
Figure 1
Sequence alignment of mouse aczonin (mACZ), chicken aczonin (cACZ), and rat Bassoon (Bsn). The long splicing variants are shown (mouse, L; chicken, XL), and the position at mouse codon 4829 is indicated by an asterisk where the QQLRIQP sequence can instead be followed by the short SKRRK COOH terminus. Overlining beginning at mouse codon 430 marks three 10-mer repeat units deleted in some mouse cDNAs. The chicken sequence is incomplete for the ∼80 NH2-terminal codons. Upstream of the putative start codon, the mouse cDNA contig continues for 304 nucleotides of GC-rich sequence with no in-frame stop codon. Additional rescreenings did not yield sequences reaching further upstream. Between aczonin and Bassoon, the first nine codons are synonymous, whereas the upstream cDNA sequences are completely dissimilar, also suggesting that the codon assumed here as methionine 1 is the true start codon. Specific sequence motifs (see Fig. 2) are framed by arrowheads above the mouse sequence (except the zinc finger and polyproline motifs that are self-evident) and designated at the right margin. The rat Bassoon sequence is taken from tom Dieck et al. 1998. EMBL/GenBank/DDBJ sequence database accession numbers are Y19185-6 (mouse aczonin-L and S), Y19187 (chicken aczonin-XL), and Y19188 (partial human aczonin; data not shown).
Figure 2
Regional organization of aczonin and partial homology to Bassoon and Rim. For aczonin, wide bars indicate sequence regions with high similarity between chicken and mouse, and narrow bars indicate sequences with low interspecies conservation. Triangles mark regions with 10-mer repeats, and ovoids mark the region with 22-mer repeats in the chicken sequence (actual repeat units are shorter and more numerous than these symbols). Black boxes represent zinc finger (Zn), polyproline (PP), PDZ, and C2 modules as indicated. Shaded boxes in Bassoon and Rim indicate additional sequence regions with similarity to aczonin. Cross-hatched boxes indicate a sequence region of particularly high conservation between mouse aczonin, chicken aczonin, and Bassoon. Regions of sequence similarity are connected by dashed lines. In aczonin, a vertical dashed line near the NH2 terminus indicates the end of the chicken sequence, and two vertical lines between the C2 modules indicate the sites of differential splicing.
Figure 3
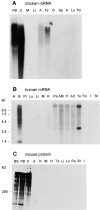
Tissue specificity of aczonin mRNA and protein expression. (A) Chicken aczonin mRNA (10 μg poly(A)+ RNA per lane). (B) Human aczonin mRNA (2 μg poly(A)+ RNA per lane). (C) Mouse aczonin protein (80 μg of tissue homogenate protein per lane). Tissue abbreviations are: A, adrenal gland; AC, adrenal cortex; AM, adrenal medulla; B, brain; BS, brain stem; C, cerebellum; FB, forebrain; H, heart; I, small intestine; K, kidney; Li, liver; Lu, lung; M, muscle; O, ovary; Pa, pancreas; Pl, placenta; Sp, spleen; St, stomach; Te, testis; Tm, thymus; and Tr, thyroid. Smears are attributed to partial degradation of these very long mRNA and protein molecules. Long exposures are shown to illustrate tissue specificity.
Figure 4
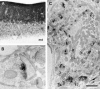
Immunohisto-chemical localization of aczonin in rat brain. Light microscopic inspection of the cerebellar cortex (A) shows finely punctate staining of the molecular layer (m), and ring-shaped or patchy immunopositive structures in the granule cell layer (g), whereas the medulla (md) is immunonegative. p indicates Purkinje cell layer. Electron microscopy shows that immunoperoxidase reaction product is restricted to the presynaptic active zones (B) of an asymmetric synapse with a dendritic spine in the molecular layer of the dentate gyrus or (C) of a mossy fiber terminal in a cerebellar glomerulus. In B, note that aczonin immunoreactivity is focused to the two junctional zones of the perforated synaptic specialization. Bar: 115 μm (A); 0.33 μm (B); or 1 μm (C).
Figure 5
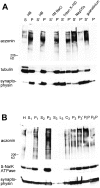
Distribution of aczonin in subcellular fractionation. (A) Mouse brain homogenate was subjected to 120,000 g fractionation (S, supernatant; P, pellet) in a detergent-free homogenization buffer (HB) containing 150 mM NaCl as described in Materials and Methods. The pellet fraction P was resuspended in the homogenization buffer (HB) or in various extraction buffers (1 M NaCl in homogenization buffer; 1% Triton X-100 in homogenization buffer without NaCl; 100 mM Na2CO3, pH 11.5; 6 M guanidinium chloride) and recentrifuged at 120,000 g. Supernatant and pellet fractions after recentrifugation are termed S′ and P′. Equal aliquots of all fractions were analyzed by SDS-PAGE and immunoblotting with aczonin, tubulin, and synaptophysin antibodies. In the experiment shown, extraction was carried out at 4°C for 20 min. The same distribution was obtained when extraction was performed at room temperature for 30 min. In additional experiments not shown, aczonin could be partially extracted from the pellet by 8 M urea, but not by 3% NP-40. (B) Synaptic vesicles were purified from rat brain according to Hell et al. 1988: H, homogenate; S1 and P1, 47,000 g supernatant and pellet derived from H; S2 and P2, 120,000 g supernatant and pellet derived from S1; supernatant S3, fluffy layer L3, cushion C3, and pellet P3 from the 260,000 g spin of S2; P3′, resuspended and cleared P3 before controlled-pore glass chromatography; PIP and PIIP, pools from breakthrough peak and vesicle peak of the controlled-pore glass chromatography. 30 μg protein was applied per lane and analyzed by immunoblotting as indicated.
Figure 6
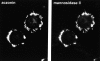
In neuronal cell lines, aczonin is associated with endomembranes. In PC12 cells as shown, double-immunofluorescence demonstrates colocalization with the Golgi complex marker, mannosidase II.
Figure 7
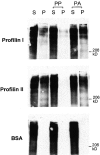
Aczonin binds profilin. Recombinant profilins I and II covalently coupled to Sepharose precipitate aczonin from mouse brain lysate (S, supernatant; P, pellet). Profilin binding is blocked by preincubation of the profilin resin with polyproline (PP), but not by polyalanine (PA). Immobilized BSA as a negative control does not precipitate aczonin. See Results for additional control experiments not shown.
Similar articles
- Rim1 and rabphilin-3 bind Rab3-GTP by composite determinants partially related through N-terminal alpha -helix motifs.
Wang X, Hu B, Zimmermann B, Kilimann MW. Wang X, et al. J Biol Chem. 2001 Aug 31;276(35):32480-8. doi: 10.1074/jbc.M103337200. Epub 2001 Jun 28. J Biol Chem. 2001. PMID: 11431472 - Piccolo, a presynaptic zinc finger protein structurally related to bassoon.
Fenster SD, Chung WJ, Zhai R, Cases-Langhoff C, Voss B, Garner AM, Kaempf U, Kindler S, Gundelfinger ED, Garner CC. Fenster SD, et al. Neuron. 2000 Jan;25(1):203-14. doi: 10.1016/s0896-6273(00)80883-1. Neuron. 2000. PMID: 10707984 - A protein interaction node at the neurotransmitter release site: domains of Aczonin/Piccolo, Bassoon, CAST, and rim converge on the N-terminal domain of Munc13-1.
Wang X, Hu B, Zieba A, Neumann NG, Kasper-Sonnenberg M, Honsbein A, Hultqvist G, Conze T, Witt W, Limbach C, Geitmann M, Danielson H, Kolarow R, Niemann G, Lessmann V, Kilimann MW. Wang X, et al. J Neurosci. 2009 Oct 7;29(40):12584-96. doi: 10.1523/JNEUROSCI.1255-09.2009. J Neurosci. 2009. PMID: 19812333 Free PMC article. - Molecular organization and assembly of the presynaptic active zone of neurotransmitter release.
Fejtova A, Gundelfinger ED. Fejtova A, et al. Results Probl Cell Differ. 2006;43:49-68. doi: 10.1007/400_012. Results Probl Cell Differ. 2006. PMID: 17068967 Review. - The presynaptic cytomatrix of brain synapses.
Dresbach T, Qualmann B, Kessels MM, Garner CC, Gundelfinger ED. Dresbach T, et al. Cell Mol Life Sci. 2001 Jan;58(1):94-116. doi: 10.1007/PL00000781. Cell Mol Life Sci. 2001. PMID: 11229820 Free PMC article. Review.
Cited by
- Profilin II is alternatively spliced, resulting in profilin isoforms that are differentially expressed and have distinct biochemical properties.
Lambrechts A, Braun A, Jonckheere V, Aszodi A, Lanier LM, Robbens J, Van Colen I, Vandekerckhove J, Fässler R, Ampe C. Lambrechts A, et al. Mol Cell Biol. 2000 Nov;20(21):8209-19. doi: 10.1128/MCB.20.21.8209-8219.2000. Mol Cell Biol. 2000. PMID: 11027290 Free PMC article. - In vivo knockdown of Piccolino disrupts presynaptic ribbon morphology in mouse photoreceptor synapses.
Regus-Leidig H, Fuchs M, Löhner M, Leist SR, Leal-Ortiz S, Chiodo VA, Hauswirth WW, Garner CC, Brandstätter JH. Regus-Leidig H, et al. Front Cell Neurosci. 2014 Sep 3;8:259. doi: 10.3389/fncel.2014.00259. eCollection 2014. Front Cell Neurosci. 2014. PMID: 25232303 Free PMC article. - Piccolo mediates EGFR signaling and acts as a prognostic biomarker in esophageal squamous cell carcinoma.
Zhang W, Hong R, Xue L, Ou Y, Liu X, Zhao Z, Xiao W, Dong D, Dong L, Fu M, Ma L, Lu N, Chen H, Song Y, Zhan Q. Zhang W, et al. Oncogene. 2017 Jul 6;36(27):3890-3902. doi: 10.1038/onc.2017.15. Epub 2017 Mar 6. Oncogene. 2017. PMID: 28263981 - ELKS1 localizes the synaptic vesicle priming protein bMunc13-2 to a specific subset of active zones.
Kawabe H, Mitkovski M, Kaeser PS, Hirrlinger J, Opazo F, Nestvogel D, Kalla S, Fejtova A, Verrier SE, Bungers SR, Cooper BH, Varoqueaux F, Wang Y, Nehring RB, Gundelfinger ED, Rosenmund C, Rizzoli SO, Südhof TC, Rhee JS, Brose N. Kawabe H, et al. J Cell Biol. 2017 Apr 3;216(4):1143-1161. doi: 10.1083/jcb.201606086. Epub 2017 Mar 6. J Cell Biol. 2017. PMID: 28264913 Free PMC article.
References
- Babitch J.A., Breithaupt T.B., Chiu T.-C., Garadi R., Helseth D.L. Preparation of chick brain synaptosomes and synaptosomal membranes. Biochim. Biophys. Acta. 1976;433:75–89. - PubMed
- Bernstein B.W., Bamburg J.R. Cycling of actin assembly in synaptosomes and neurotransmitter release. Neuron. 1989;3:257–265. - PubMed
- Bernstein B.W., DeWit M., Bamburg J.R. Actin disassembles reversibly during electrically induced recycling of synaptic vesicles in cultured neurons. Mol. Brain Res. 1998;53:236–250. - PubMed
- Burns M.E., Augustine G.J. Synaptic structure and functiondynamic organization yields architectural precision. Cell. 1995;83:187–194. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous