Cell wall stress depolarizes cell growth via hyperactivation of RHO1 - PubMed (original) (raw)
Cell wall stress depolarizes cell growth via hyperactivation of RHO1
P A Delley et al. J Cell Biol. 1999.
Abstract
Cells sense and physiologically respond to environmental stress via signaling pathways. Saccharomyces cerevisiae cells respond to cell wall stress by transiently depolarizing the actin cytoskeleton. We report that cell wall stress also induces a transient depolarized distribution of the cell wall biosynthetic enzyme glucan synthase FKS1 and its regulatory subunit RHO1, possibly as a mechanism to repair general cell wall damage. The redistribution of FKS1 is dependent on the actin cytoskeleton. Depolarization of the actin cytoskeleton and FKS1 is mediated by the plasma membrane protein WSC1, the RHO1 GTPase switch, PKC1, and a yet-to-be defined PKC1 effector branch. WSC1 behaves like a signal transducer or a stress-specific actin landmark that both controls and responds to the actin cytoskeleton, similar to the bidirectional signaling between integrin receptors and the actin cytoskeleton in mammalian cells. The PKC1-activated mitogen-activated protein kinase cascade is not required for depolarization, but rather for repolarization of the actin cytoskeleton and FKS1. Thus, activated RHO1 can mediate both polarized and depolarized cell growth via the same effector, PKC1, suggesting that RHO1 may function as a rheostat rather than as a simple on-off switch.
Figures
Figure 1
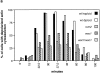
WSC1 and ROM2 are required for heat-induced depolarization of the actin cytoskeleton. (a) Wild-type (wt) (JK9-3da), rom2 (AS138-1b), and wsc1 (PA39-1b) haploid cells, and wild-type (JK9-3da/α) and wsc1/wsc1 (PA96) diploid cells were grown in rich medium at 24°C, shifted to 37°C for the indicated time (in minutes), fixed, and processed for visualization of the actin cytoskeleton. The percentage of cells (n > 200) exhibiting depolarized actin patches was determined (see Materials and Methods). A representative of three experiments is shown. (b) Wild-type (JK9-3da) (A–D) and rom2 (AS138-1b) (E–H) haploid cells, and wild-type (JK9-3da/α) (I–L) and wsc1/wsc1 (PA96) (M-P) diploid cells were grown in rich medium at 24°C, shifted to 37°C for 35 min, fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, and O) and Nomarski (B, D, F, H, J, L, N, and P) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
Figure 1
WSC1 and ROM2 are required for heat-induced depolarization of the actin cytoskeleton. (a) Wild-type (wt) (JK9-3da), rom2 (AS138-1b), and wsc1 (PA39-1b) haploid cells, and wild-type (JK9-3da/α) and wsc1/wsc1 (PA96) diploid cells were grown in rich medium at 24°C, shifted to 37°C for the indicated time (in minutes), fixed, and processed for visualization of the actin cytoskeleton. The percentage of cells (n > 200) exhibiting depolarized actin patches was determined (see Materials and Methods). A representative of three experiments is shown. (b) Wild-type (JK9-3da) (A–D) and rom2 (AS138-1b) (E–H) haploid cells, and wild-type (JK9-3da/α) (I–L) and wsc1/wsc1 (PA96) (M-P) diploid cells were grown in rich medium at 24°C, shifted to 37°C for 35 min, fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, and O) and Nomarski (B, D, F, H, J, L, N, and P) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
Figure 2
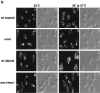
The actin cytoskeleton is required for de- and repolarization of glucan synthase upon heat shock. (a) Wild-type cells (JK9-3da) were grown at 24°C, shifted at 37°C for the indicated time, fixed, and processed for detection of FKS1 by immunofluorescence (see Materials and Methods). LAT-A was added at time of shift to 37°C (E–F) and after 35 min at 37°C (I–J). Fluorescence (A, C, E, G, and I, FKS1) and Nomarski (B, D, F, H, and J) images are shown. (b) Cells expressing HA-RHO1 (PA120-3b) were grown in rich medium at 24°C, shifted to 37°C for the indicated time, fixed, and processed for detection of HA-RHO1 by immunofluorescence (see Materials and Methods). Fluorescence (A, C, E, G, I, and K, RHO1) and Nomarski (B, D, F, H, J, and L) images are shown.
Figure 3
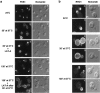
Hyperactivated RHO1 or PKC1 is sufficient to induce actin depolarization. Wild-type (JK9-3da) cells carrying an empty vector (A–D), pGAL-RHO1* (E–H), pGAL-PKC1* (I–L), pGAL-BCK1* (M–P), or pGAL-MKK1* (Q–T) were grown at 30°C in minimal medium containing raffinose. Galactose was added to a final concentration of 2% and strains were further incubated for 2.5 h (empty vector, pGAL-RHO1* or pGAL-PKC1*) or 5 h (pGAL-BCK1* or pGAL-MKK1*). Cells containing pGAL-BCK1* or pGAL-MKK1* gave the same result when incubated in galactose for 2.5 or 5 h. Samples were fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, O, Q, and S) and Nomarski (B, D, F, H, J, L, N, P, R, and T) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
Figure 4
BCK1 and MPK1 are required for repolarization of the actin cytoskeleton upon heat shock. Wild-type (A–F, JK9-3da), bck1 (G–L, PA109-1c), and mpk1 (M–R, TS45-1a) cells were grown in rich medium at 24°C and shifted to 37°C for 35 and 180 min. Samples were fixed, stained with TRITC-phalloidin, and observed by fluorescence (A, C, E, G, I, K, M, O, and Q) and Nomarski (B, D, F, H, J, L, N, P, and R) microscopy to visualize the actin cytoskeleton and whole cells, respectively.
Figure 5
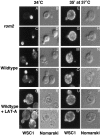
Stress-induced depolarization of WSC1 distribution is independent of a depolarized actin cytoskeleton, yet WSC1 localization is controlled by the actin cytoskeleton. Wild-type (I–X, JK9-3da) or rom2 (A–H, AS138-1b) cells expressing WSC1-HA (pWSC1-HA) were grown in minimal medium at 24°C, shifted to 37°C for 35 min or maintained at 24°C, fixed, and processed for detection of WSC1-HA by immunofluorescence (see Materials and Methods). In cells grown continuously at 24°C, LAT-A was added 35 min before fixation (Q–T). In cells shifted to 37°C, LAT-A was added at the time of shift (U–X). Fluorescence (A, C, E, G, I, K, M, O, Q, S, U, and W) and Nomarski (B, D, F, H, J, L, N, P, R, T, V, and X) images are shown.
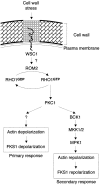
Figure 6
Model for the regulation of actin cytoskeleton and FKS1 distribution upon cell wall stress (see Discussion for further details).
Similar articles
- The Rho1 effector Pkc1, but not Bni1, mediates signalling from Tor2 to the actin cytoskeleton.
Helliwell SB, Schmidt A, Ohya Y, Hall MN. Helliwell SB, et al. Curr Biol. 1998 Nov 5;8(22):1211-4. doi: 10.1016/s0960-9822(07)00511-8. Curr Biol. 1998. PMID: 9811607 - Yeast protein kinases and the RHO1 exchange factor TUS1 are novel components of the cell integrity pathway in yeast.
Schmelzle T, Helliwell SB, Hall MN. Schmelzle T, et al. Mol Cell Biol. 2002 Mar;22(5):1329-39. doi: 10.1128/MCB.22.5.1329-1339.2002. Mol Cell Biol. 2002. PMID: 11839800 Free PMC article. - A MAP kinase dependent feedback mechanism controls Rho1 GTPase and actin distribution in yeast.
Guo S, Shen X, Yan G, Ma D, Bai X, Li S, Jiang Y. Guo S, et al. PLoS One. 2009 Jun 30;4(6):e6089. doi: 10.1371/journal.pone.0006089. PLoS One. 2009. PMID: 19564916 Free PMC article. - Cell wall integrity signaling in Saccharomyces cerevisiae.
Levin DE. Levin DE. Microbiol Mol Biol Rev. 2005 Jun;69(2):262-91. doi: 10.1128/MMBR.69.2.262-291.2005. Microbiol Mol Biol Rev. 2005. PMID: 15944456 Free PMC article. Review. - The TORC2-Dependent Signaling Network in the Yeast Saccharomyces cerevisiae.
Roelants FM, Leskoske KL, Martinez Marshall MN, Locke MN, Thorner J. Roelants FM, et al. Biomolecules. 2017 Sep 5;7(3):66. doi: 10.3390/biom7030066. Biomolecules. 2017. PMID: 28872598 Free PMC article. Review.
Cited by
- Determinants of Swe1p degradation in Saccharomyces cerevisiae.
McMillan JN, Theesfeld CL, Harrison JC, Bardes ES, Lew DJ. McMillan JN, et al. Mol Biol Cell. 2002 Oct;13(10):3560-75. doi: 10.1091/mbc.e02-05-0283. Mol Biol Cell. 2002. PMID: 12388757 Free PMC article. - Rvs161p and sphingolipids are required for actin repolarization following salt stress.
Balguerie A, Bagnat M, Bonneu M, Aigle M, Breton AM. Balguerie A, et al. Eukaryot Cell. 2002 Dec;1(6):1021-31. doi: 10.1128/EC.1.6.1021-1031.2002. Eukaryot Cell. 2002. PMID: 12477802 Free PMC article. - Sec3p is needed for the spatial regulation of secretion and for the inheritance of the cortical endoplasmic reticulum.
Wiederkehr A, Du Y, Pypaert M, Ferro-Novick S, Novick P. Wiederkehr A, et al. Mol Biol Cell. 2003 Dec;14(12):4770-82. doi: 10.1091/mbc.e03-04-0229. Epub 2003 Sep 5. Mol Biol Cell. 2003. PMID: 12960429 Free PMC article. - Local Anesthetics and Antipsychotic Phenothiazines Interact Nonspecifically with Membranes and Inhibit Hexose Transporters in Yeast.
Uesono Y, Toh-e A, Kikuchi Y, Araki T, Hachiya T, Watanabe CK, Noguchi K, Terashima I. Uesono Y, et al. Genetics. 2016 Mar;202(3):997-1012. doi: 10.1534/genetics.115.183806. Epub 2016 Jan 12. Genetics. 2016. PMID: 26757771 Free PMC article. - A new cell cycle checkpoint that senses plasma membrane/cell wall damage in budding yeast.
Kono K, Ikui AE. Kono K, et al. Bioessays. 2017 Apr;39(4):10.1002/bies.201600210. doi: 10.1002/bies.201600210. Epub 2017 Feb 17. Bioessays. 2017. PMID: 28211950 Free PMC article. Review.
References
- Alberts A.S., Bouquin N., Johnston L.H., Treisman R. Analysis of RhoA-binding proteins reveals an interaction domain conserved in heterotrimeric G protein beta subunits and the yeast response regulator protein Skn7. J. Biol. Chem. 1998;273:8616–8622. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases