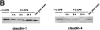Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier - PubMed (original) (raw)
Clostridium perfringens enterotoxin fragment removes specific claudins from tight junction strands: Evidence for direct involvement of claudins in tight junction barrier
N Sonoda et al. J Cell Biol. 1999.
Abstract
Claudins, comprising a multigene family, constitute tight junction (TJ) strands. Clostridium perfringens enterotoxin (CPE), a single approximately 35-kD polypeptide, was reported to specifically bind to claudin-3/RVP1 and claudin-4/CPE-R at its COOH-terminal half. We examined the effects of the COOH-terminal half fragment of CPE (C-CPE) on TJs in L transfectants expressing claudin-1 to -4 (C1L to C4L, respectively), and in MDCK I cells expressing claudin-1 and -4. C-CPE bound to claudin-3 and -4 with high affinity, but not to claudin-1 or -2. In the presence of C-CPE, reconstituted TJ strands in C3L cells gradually disintegrated and disappeared from their cell surface. In MDCK I cells incubated with C-CPE, claudin-4 was selectively removed from TJs with its concomitant degradation. At 4 h after incubation with C-CPE, TJ strands were disintegrated, and the number of TJ strands and the complexity of their network were markedly decreased. In good agreement with the time course of these morphological changes, the TJ barrier (TER and paracellular flux) of MDCK I cells was downregulated by C-CPE in a dose-dependent manner. These findings provided evidence for the direct involvement of claudins in the barrier functions of TJs.
Figures
Figure 1
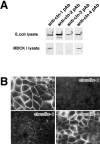
Claudins expressed in MDCK I cells. (A) Immunoblotting. The total lysate of E. coli (E. coli lysate) expressing MBP fusion protein with the cytoplasmic domains of claudin-1 (1) and GST fusion proteins with the cytoplasmic domains of claudin-2 (2), -3 (3), and -4 (4), and the total lysate of MDCK I cells (MDCK I lysate) were separated by SDS-PAGE, followed by immunoblotting with anti–claudin-1 pAb (anti-cln-1 pAb), anti–claudin-2 pAb (anti-cln-2 pAb), anti–claudin-3 pAb (anti-cln-3 pAb), and anti–claudin-4 pAb (anti-cln-4 pAb). Only claudin-1 and -4 were detected in MDCK I cells. (B) Immunofluorescence microscopy. Confluent cultures of MDCK I cells were stained with anti–claudin-1 pAb (claudin-1), anti–claudin-2 pAb (claudin-2), anti–claudin-3 pAb (claudin-3), or anti–claudin-4 pAb (claudin-4). Both claudin-1 and -4 were concentrated at cell–cell borders in large amounts, where only a trace amount of claudin-3 was also detected. Claudin-2 signal was undetectable. Bar, 20 μm.
Figure 2
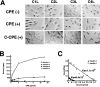
Specific interaction of Clostridium perfringens enterotoxin (CPE) with claudin-3 and -4. (A) L transfectants expressing claudin-1 (C1L), claudin-2 (C2L), claudin-3 (C3L), or claudin-4 (C4L) were cultured in the absence (CPE(−)) or presence of 500 ng/ml full-length CPE (CPE(+)) or 2.5 μg/ml COOH-terminal half fragment of CPE (C-CPE(+)). Phase-contrast microscopic images taken at 1 h after incubation revealed that CPE, but not C-CPE, showed cytotoxicity only to C3L and C4L cells. Bar, 20 μm. (B) L transfectants (1 × 105 cells) expressing claudin-1, -2, -3, or -4 were incubated with various concentration of 125I-CPE, and binding was determined as reported previously (Katahira et al. 1997a). The binding of 125I-CPE to claudin-1 or -2 was negligible. Each symbol represents mean value (n = 6). (C) Scatchard plot of the specific binding of 125I-CPE to L transfectants expressing claudin-3 (closed triangles; _K_a = 8.4 × 107 M−1) or claudin-4 (open circles; _K_a = 1.1 × 108 M−1).
Figure 3
Effects of C-CPE on the subcellular distribution of claudin-3 and the morphology of reconstituted TJ strands in L transfectants expressing claudin-3 (C3L cells). (a and b) Immunofluorescence microscopy. When C3L cells were stained with anti–claudin-3 pAb (a), claudin-3 was shown to be concentrated at cell–cell borders as planes (arrows). At 4 h after incubation with 2.5 μg/ml C-CPE, these claudin-3-positive planes were fragmented into punctate structures and gradually disappeared (b). (c and d) Freeze-fracture replica electron microscopy. In the absence of C-CPE, a well-developed network of TJ strands was reconstituted between adjacent C3L cells (c). When cells were incubated with C-CPE for 4 h, TJ strands were disintegrated into fragmented belt-like aggregates of intramembranous particles (d). Bars: (a and b) 20 μm; (c and d) 200 nm.
Figure 4
Selective loss of claudin-4 from TJs in MDCK I cells. (A) Immunofluorescence microscopy. At 0 (−C-CPE), 4, 8, or 24 h after incubation with 2.5 μg/ml C-CPE in the basolateral compartment or at 24 h after C-CPE was removed, MDCK I cells plated at confluent density on filters were double stained with anti–claudin-1 pAb (claudin-1) and anti–claudin-4 pAb (claudin-4). C-CPE did not affect the subcellular distribution of claudin-1. At 4 h after incubation with C-CPE, claudin-4 was distributed in the cytoplasm with concomitant gradual disappearance from cell–cell borders. At 24 h after incubation, the claudin-4 signal was undetectable, but when C-CPE was washed out at this time point, the concentration of claudin-4 at the junctional region was recovered completely. Bar, 40 μm. (B) Immunoblotting. Total cell lysates of MDCK I cells were separated by SDS-PAGE, followed by immunoblotting with anti–claudin-1 pAb (claudin-1) or anti–claudin-4 pAb (claudin-4). In the presence of C-CPE, claudin-4, but not claudin-1, levels decreased with a similar time course to the disappearance of the immunofluorescence signal of claudin-4 (see A).
Figure 4
Selective loss of claudin-4 from TJs in MDCK I cells. (A) Immunofluorescence microscopy. At 0 (−C-CPE), 4, 8, or 24 h after incubation with 2.5 μg/ml C-CPE in the basolateral compartment or at 24 h after C-CPE was removed, MDCK I cells plated at confluent density on filters were double stained with anti–claudin-1 pAb (claudin-1) and anti–claudin-4 pAb (claudin-4). C-CPE did not affect the subcellular distribution of claudin-1. At 4 h after incubation with C-CPE, claudin-4 was distributed in the cytoplasm with concomitant gradual disappearance from cell–cell borders. At 24 h after incubation, the claudin-4 signal was undetectable, but when C-CPE was washed out at this time point, the concentration of claudin-4 at the junctional region was recovered completely. Bar, 40 μm. (B) Immunoblotting. Total cell lysates of MDCK I cells were separated by SDS-PAGE, followed by immunoblotting with anti–claudin-1 pAb (claudin-1) or anti–claudin-4 pAb (claudin-4). In the presence of C-CPE, claudin-4, but not claudin-1, levels decreased with a similar time course to the disappearance of the immunofluorescence signal of claudin-4 (see A).
Figure 5
Freeze-fracture replica images of TJs in MDCK I cells incubated with C-CPE. At 0, 4, or 8 h after incubation with 2.5 μg/ml C-CPE in the basolateral compartment, MDCK I cells plated at confluent density on filters were fixed with glutaraldehyde, and processed for freeze-fracture replica electron microscopy. In the absence of C-CPE (0 H), MDCK I cells were characterized by well-developed anastomosing networks of TJ strands. At 4 h after incubation with C-CPE (4 H), TJ strands facing toward the basolateral membrane domains began to disintegrate to thick belt-like aggregates of intramembranous particles (arrows). Around 8 h after incubation (8 H), these belt-like particle aggregates had mostly disappeared, leaving a fairly simple TJ strand network. Bar, 20 μm.
Figure 6
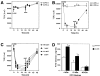
Effects of C-CPE on the TJ barrier function of MDCK I cells. (A–C) TER measurements. MDCK I cells were plated at confluent density on 12-mm filters. When 2.5 μg/ml C-CPE was added to the apical compartment at t = 0 (C-CPE), the TER was not affected but remained at the level of 8,000–10,000 Ωcm2 (A; n = 10 for each condition). Addition of C-CPE (2.5 μg/ml) in the basolateral compartment (at t = 0; C-CPE) resulted in an ∼4.5-fold reduction in TER from ∼9,000 Ωcm2 to ∼2,000 Ωcm2 within 4 h, and removal of C-CPE from the compartment (at t = 24 h; wash) induced gradual recovery of TER to the level of nontreated cells within one day (B; n = 10 for each condition). In B, asterisks denote significant difference from nonincubated cells at the corresponding time point (P < 0.01). The C-CPE–induced reduction of TER was dose-dependent (C; n = 4 for each condition). (D) Paracellular tracer flux assay (n = 4 for each condition). C-CPE (2.5 μg/ml) in the basolateral compartment caused an approximately twofold increase in the flux of FITC-dextran 4K and 10K (*P < 0.01), but not 40K. All error bars represent standard deviations.
Similar articles
- Structural basis for Clostridium perfringens enterotoxin targeting of claudins at tight junctions in mammalian gut.
Vecchio AJ, Rathnayake SS, Stroud RM. Vecchio AJ, et al. Proc Natl Acad Sci U S A. 2021 Apr 13;118(15):e2024651118. doi: 10.1073/pnas.2024651118. Proc Natl Acad Sci U S A. 2021. PMID: 33876770 Free PMC article. - Role of C-terminal regions of the C-terminal fragment of Clostridium perfringens enterotoxin in its interaction with claudin-4.
Takahashi A, Kondoh M, Masuyama A, Fujii M, Mizuguchi H, Horiguchi Y, Watanabe Y. Takahashi A, et al. J Control Release. 2005 Nov 2;108(1):56-62. doi: 10.1016/j.jconrel.2005.07.008. Epub 2005 Aug 8. J Control Release. 2005. PMID: 16091298 - Manner of interaction of heterogeneous claudin species within and between tight junction strands.
Furuse M, Sasaki H, Tsukita S. Furuse M, et al. J Cell Biol. 1999 Nov 15;147(4):891-903. doi: 10.1083/jcb.147.4.891. J Cell Biol. 1999. PMID: 10562289 Free PMC article. - Disruption of Claudin-Made Tight Junction Barriers by Clostridium perfringens Enterotoxin: Insights from Structural Biology.
Ogbu CP, Roy S, Vecchio AJ. Ogbu CP, et al. Cells. 2022 Mar 5;11(5):903. doi: 10.3390/cells11050903. Cells. 2022. PMID: 35269525 Free PMC article. Review. - [Non-invasive drug delivery system with the claudin binder].
Takahashi A, Kondoh M, Yagi K. Takahashi A, et al. Yakugaku Zasshi. 2011;131(11):1583-7. doi: 10.1248/yakushi.131.1583. Yakugaku Zasshi. 2011. PMID: 22041696 Review. Japanese.
Cited by
- M cell targeting by a Claudin 4 targeting peptide can enhance mucosal IgA responses.
Lo DD, Ling J, Eckelhoefer AH. Lo DD, et al. BMC Biotechnol. 2012 Mar 13;12:7. doi: 10.1186/1472-6750-12-7. BMC Biotechnol. 2012. PMID: 22413871 Free PMC article. - Distinct claudin expression profiles of hepatocellular carcinoma and metastatic colorectal and pancreatic carcinomas.
Holczbauer Á, Gyöngyösi B, Lotz G, Szijártó A, Kupcsulik P, Schaff Z, Kiss A. Holczbauer Á, et al. J Histochem Cytochem. 2013 Apr;61(4):294-305. doi: 10.1369/0022155413479123. Epub 2013 Feb 5. J Histochem Cytochem. 2013. PMID: 23385421 Free PMC article. - Critical role of tight junctions in drug delivery across epithelial and endothelial cell layers.
González-Mariscal L, Nava P, Hernández S. González-Mariscal L, et al. J Membr Biol. 2005 Sep;207(2):55-68. doi: 10.1007/s00232-005-0807-y. J Membr Biol. 2005. PMID: 16477528 Review. - Size-selective loosening of the blood-brain barrier in claudin-5-deficient mice.
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S. Nitta T, et al. J Cell Biol. 2003 May 12;161(3):653-60. doi: 10.1083/jcb.200302070. J Cell Biol. 2003. PMID: 12743111 Free PMC article. - Mucosal healing and inflammatory bowel disease: Therapeutic implications and new targets.
Otte ML, Lama Tamang R, Papapanagiotou J, Ahmad R, Dhawan P, Singh AB. Otte ML, et al. World J Gastroenterol. 2023 Feb 21;29(7):1157-1172. doi: 10.3748/wjg.v29.i7.1157. World J Gastroenterol. 2023. PMID: 36926666 Free PMC article. Review.
References
- Anderson J.M., Van Itallie C.M. Tight junctions and the molecular basis for regulation of paracellular permeability. Am. J. Physiol. 1995;269:G467–G475. - PubMed
- Balda M.S., Whitney J.A., Flores C., González S., Cereijido M., Matter K. Functional dissociation of paracellular permeability and transepithelial electrical resistance and disruption of the apical-basolateral intramembrane diffusion barrier by expression of a mutant tight junction membrane protein. J. Cell Biol. 1996;134:1031–1049. - PMC - PubMed
- Briehl M.M., Miesfeld R.L. Isolation and characterization of transcripts induced by androgen withdrawal and apoptotic cell death in the rat ventral prostate. Mol. Endocrinol. 1991;5:1381–1388. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous