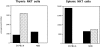A defect in interleukin 12-induced activation and interferon gamma secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus - PubMed (original) (raw)
A defect in interleukin 12-induced activation and interferon gamma secretion of peripheral natural killer T cells in nonobese diabetic mice suggests new pathogenic mechanisms for insulin-dependent diabetes mellitus
M Falcone et al. J Exp Med. 1999.
Abstract
The function of natural killer T (NKT) cells in the immune system has yet to be determined. There is some evidence that their defect is associated with autoimmunity, but it is still unclear how they play a role in regulating the pathogenesis of T cell-mediated autoimmune diseases. It was originally proposed that NKT cells could control autoimmunity by shifting the cytokine profile of autoimmune T cells toward a protective T helper 2 cell (Th2) type. However, it is now clear that the major function of NKT cells in the immune system is not related to their interleukin (IL)-4 secretion. In fact, NKT cells mainly secrete interferon (IFN)-gamma and, activated in the presence of IL-12, acquire a strong inflammatory phenotype and cytotoxic function.
Figures
Figure 1
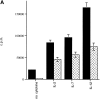
The proliferative response to TCR plus IL-12–mediated stimulation is affected in peripheral NKT cells of NOD mice. (A) The NKT cells were stained with the cocktail of three antibodies against markers of T cells (anti-CD3–PE) and NK cells (anti-Ly49A–cychrome and anti–IL-2 receptor β chain–FITC) and purified by FACS® sorting. Freshly sorted NKT cells from splenocytes of NOD and C57BL/6 mice were in vitro stimulated in TCR-β–bound 96-well plates. IL-2 alone or with IL-7 or IL-12 was added to the cultures to measure their effect on TCR-mediated stimulation of NKT cells. The degree of proliferation was measured by [3H]thymidine incorporation and expressed as cpm ± SD. NKT cells of NOD (hatched bars) mice showed a reduced proliferation with or without the addition of cytokines to the cultures compared with the same T cell population isolated from spleens of age-matched C57BL/6 mice (black bars). The impaired proliferation was even more striking in the NOD NKT cell cultures stimulated with IL-12. Data are from one representative experiment out of three and represent the geometric mean of duplicate determinations. (B) NKT cells sorted from NOD or C57BL/6 splenocytes were cultured in anti–TCR-β bound 24-wells plate in the presence of IL-12. The number of peripheral NKT cells found in spleens of NOD mice was lower than that of C57BL/6 mice (black bars). The NKT cell number difference became more evident after TCR plus IL-12–mediated stimulation. While NKT cells of C57BL/6 expanded significantly after 4 d of culture (50% increase), NKT cells of NOD mice did not amplify in culture (hatched bars).
Figure 1
The proliferative response to TCR plus IL-12–mediated stimulation is affected in peripheral NKT cells of NOD mice. (A) The NKT cells were stained with the cocktail of three antibodies against markers of T cells (anti-CD3–PE) and NK cells (anti-Ly49A–cychrome and anti–IL-2 receptor β chain–FITC) and purified by FACS® sorting. Freshly sorted NKT cells from splenocytes of NOD and C57BL/6 mice were in vitro stimulated in TCR-β–bound 96-well plates. IL-2 alone or with IL-7 or IL-12 was added to the cultures to measure their effect on TCR-mediated stimulation of NKT cells. The degree of proliferation was measured by [3H]thymidine incorporation and expressed as cpm ± SD. NKT cells of NOD (hatched bars) mice showed a reduced proliferation with or without the addition of cytokines to the cultures compared with the same T cell population isolated from spleens of age-matched C57BL/6 mice (black bars). The impaired proliferation was even more striking in the NOD NKT cell cultures stimulated with IL-12. Data are from one representative experiment out of three and represent the geometric mean of duplicate determinations. (B) NKT cells sorted from NOD or C57BL/6 splenocytes were cultured in anti–TCR-β bound 24-wells plate in the presence of IL-12. The number of peripheral NKT cells found in spleens of NOD mice was lower than that of C57BL/6 mice (black bars). The NKT cell number difference became more evident after TCR plus IL-12–mediated stimulation. While NKT cells of C57BL/6 expanded significantly after 4 d of culture (50% increase), NKT cells of NOD mice did not amplify in culture (hatched bars).
Figure 3
IL-12 did not induce IFN-γ secretion in NKT cells of NOD mice. We analyzed the ability of IL-12 to induce IFN-γ secretion in peripheral NKT cells of NOD mice. NKT cells from total splenocytes of C57BL/6 and NOD mice were isolated by FACS® sorting and cultured with repeated anti-TCR stimulations in the presence of IL-2 with IL-7 (black bars) or IL-12 (hatched bars). The supernatants of the NKT cell cultures were collected 48 h after each restimulation and analyzed by IFN-γ and IL-4 ELISA assays. (A) Already at the first stimulation, NKT cells of C57BL/6 mice secreted IFN-γ in larger amounts than IL-4, particularly when IL-12 was added to the cultures. Noticeably, IL-12 was able to inhibit IL-4 secretion by NKT cells. NKT cells of NOD mice responded less intensively to IL-12 and secreted lower amounts of IFN-γ. (B) The defect of IL-12–induced IFN-γ secretion in NKT cells of NOD mice became more evident after continuous in vitro stimulations. At the third restimulation, while NKT cells from normal mice secreted ∼500 ng of IFN-γ per milliliter of supernatant, the same cells from NOD mice did not increase their IFN-γ secretion at all. These data are from one representative experiment out of three.
Figure 2
NKT cells of NOD mice failed to differentiate toward an IFN-γ–secreting phenotype in the periphery. The cytokine profile of NKT cells in the thymi and spleens of normal C57BL/6 and NOD mice was evaluated by IFN-γ and IL-4 ELISA assays. Freshly sorted NKT cells were briefly stimulated in vitro by plate-bound anti-TCR antibody, and supernatants were analyzed for IFN-γ (black bars) and IL-4 secretion (hatched bars). Normal NKT cells of C57BL/6 mice in the thymus secrete a larger amount of IL-4 than IFN-γ. Once in the periphery, their cytokine phenotype changed, and they differentiated toward a strongly biased IFN-γ–secreting phenotype with almost undetectable IL-4 secretion. Peripheral NKT cells of NOD mice fail to acquire the IFN-γ-secreting phenotype and they do not increase their IFN-γ secretion; however, at the same time, they retain the ability to secrete IL-4.
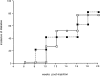
Figure 5
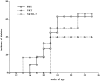
Peripheral NKT cells of NOD mice can only mediate incomplete protection against IDDM. The NKT cell repertoire of 3-wk-old NOD mice was significantly enlarged by intravenous injection of 3 × 105 NKT cells (CD3+, CD122+, and Ly49A+ cells) purified by FACS® sorting from spleens of 8–10-wk-old NOD donors. Some of the freshly isolated NKT cells were cultured for 3 d in the presence of IL-7 to restore a normal IL-4 secretion. A control group received phosphate buffer solution. Blood glucose levels were measured weekly starting at 10 wk of age. Mice with blood glucose values ≥250 mg/dl were considered diabetic. Starting at 16 wk of age, the PBS-injected controls (□; n = 11) as well as the mice that received IL-7–stimulated NKT cells (○; n = 6) developed diabetes with a similar time course. Mice that received a large number of peripheral NKT cells started developing diabetes later and with a lower incidence (▵; n = 7). However, at 22 wk of age, 42% of the NKT-injected mice had developed diabetes, and the difference in diabetes incidence compared with the control group was not statistically significant (P > 0.05) at any time point on the age-related curve. The data are cumulative from three different experiments.
Figure 4
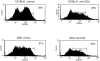
Lack of rapid turnover in peripheral NKT cells of NOD mice. The homeostasis of peripheral NKT cells was evaluated by measuring CD3+ lymphocytes in the liver of C57BL/6 and age-matched NOD mice injected intravenously with anti-CD3 mAb. Control mice were injected with PBS. After 20 h, the lymphocyte population of the liver was isolated and analyzed by FACS® sorting. In C57BL/6 mice, anti-CD3 treatment reduced the CD3+ cells in the liver ∼50% (from 62.3 to 31.7% lymphocytes), a reduction that can be integrally related to NKT cell depletion. On the contrary, the same T cell population in the liver of NOD mice showed only a slight decrease (from 52.6 to 48%). The data are from one representative experiment out of two.
Figure 6
NKT cells of NOD mice had no immunomodulatory effect on the effector phase of IDDM. To test if NKT cells from NOD mice could have any immunoregulatory role on diabetogenic effector T cells, we transferred diabetogenic splenocytes from NOD mice (14-wk-old) to NOD/Scid recipients with or without NKT cells. In brief, total splenocytes were stained for the three NKT cell markers (CD3, CD122, and Ly49A) and FACS® sorted. The NKT cell–negative splenocytes were then injected in NOD/Scid recipients with or without 6 × 105 triple-positive NKT cells. The NOD/Scid mice that received NKT cells together with the diabetogenic splenocytes (▪; n = 4) showed the same clinical course and incidence of diabetes as the mice that were injected with NKT cell–depleted splenocytes (□; n = 4). The data are cumulative from two different experiments.
Similar articles
- The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice.
Cardell SL. Cardell SL. Clin Exp Immunol. 2006 Feb;143(2):194-202. doi: 10.1111/j.1365-2249.2005.02942.x. Clin Exp Immunol. 2006. PMID: 16412042 Free PMC article. Review. - Up-regulation of CD1d expression restores the immunoregulatory function of NKT cells and prevents autoimmune diabetes in nonobese diabetic mice.
Falcone M, Facciotti F, Ghidoli N, Monti P, Olivieri S, Zaccagnino L, Bonifacio E, Casorati G, Sanvito F, Sarvetnick N. Falcone M, et al. J Immunol. 2004 May 15;172(10):5908-16. doi: 10.4049/jimmunol.172.10.5908. J Immunol. 2004. PMID: 15128771 - Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes.
Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM, Leite-De-Moraes M, Gouarin C, Zhu R, Hameg A, Nakayama T, Taniguchi M, Lepault F, Lehuen A, Bach JF, Herbelin A. Sharif S, et al. Nat Med. 2001 Sep;7(9):1057-62. doi: 10.1038/nm0901-1057. Nat Med. 2001. PMID: 11533711 - Inhibition of T helper cell type 2 cell differentiation and immunoglobulin E response by ligand-activated Valpha14 natural killer T cells.
Cui J, Watanabe N, Kawano T, Yamashita M, Kamata T, Shimizu C, Kimura M, Shimizu E, Koike J, Koseki H, Tanaka Y, Taniguchi M, Nakayama T. Cui J, et al. J Exp Med. 1999 Sep 20;190(6):783-92. doi: 10.1084/jem.190.6.783. J Exp Med. 1999. PMID: 10499917 Free PMC article. - Regulation of immune responses by natural killer T cells.
Sharif S, Delovitch TL. Sharif S, et al. Arch Immunol Ther Exp (Warsz). 2001;49 Suppl 1:S23-31. Arch Immunol Ther Exp (Warsz). 2001. PMID: 11603866 Review.
Cited by
- Activation and Regulation of B Cell Responses by Invariant Natural Killer T Cells.
Doherty DG, Melo AM, Moreno-Olivera A, Solomos AC. Doherty DG, et al. Front Immunol. 2018 Jun 18;9:1360. doi: 10.3389/fimmu.2018.01360. eCollection 2018. Front Immunol. 2018. PMID: 29967611 Free PMC article. Review. - Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis.
Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Jahng AW, et al. J Exp Med. 2001 Dec 17;194(12):1789-99. doi: 10.1084/jem.194.12.1789. J Exp Med. 2001. PMID: 11748280 Free PMC article. - The natural killer T lymphocyte: a player in the complex regulation of autoimmune diabetes in non-obese diabetic mice.
Cardell SL. Cardell SL. Clin Exp Immunol. 2006 Feb;143(2):194-202. doi: 10.1111/j.1365-2249.2005.02942.x. Clin Exp Immunol. 2006. PMID: 16412042 Free PMC article. Review. - Activation of CD1d-restricted T cells protects NOD mice from developing diabetes by regulating dendritic cell subsets.
Naumov YN, Bahjat KS, Gausling R, Abraham R, Exley MA, Koezuka Y, Balk SB, Strominger JL, Clare-Salzer M, Wilson SB. Naumov YN, et al. Proc Natl Acad Sci U S A. 2001 Nov 20;98(24):13838-43. doi: 10.1073/pnas.251531798. Epub 2001 Nov 13. Proc Natl Acad Sci U S A. 2001. PMID: 11707602 Free PMC article. - Impaired SLAM-SLAM homotypic interaction between invariant NKT cells and dendritic cells affects differentiation of IL-4/IL-10-secreting NKT2 cells in nonobese diabetic mice.
Baev DV, Caielli S, Ronchi F, Coccia M, Facciotti F, Nichols KE, Falcone M. Baev DV, et al. J Immunol. 2008 Jul 15;181(2):869-77. doi: 10.4049/jimmunol.181.2.869. J Immunol. 2008. PMID: 18606638 Free PMC article.
References
- Vicari A.P., Zlotnik A. Mouse NK1.1+ T cellsa new family of T cells. Immunol. Today. 1996;17:71–76. - PubMed
- Mieza M.A., Itoh T., Cui J.Q., Makino Y., Kawano T., Tsuchida K., Koike T., Shirai T., Yagita H., Matsuzawa A. Selective reduction of Vα14+ NK T cells associated with disease development in autoimmune-prone mice. J. Immunol. 1996;156:4035–4040. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical