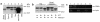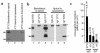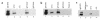Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line - PubMed (original) (raw)
Bidirectional FcRn-dependent IgG transport in a polarized human intestinal epithelial cell line
B L Dickinson et al. J Clin Invest. 1999 Oct.
Abstract
The MHC class I-related Fc receptor, FcRn, mediates the intestinal absorption of maternal IgG in neonatal rodents and the transplacental transport of maternal IgG in humans by receptor-mediated transcytosis. In mice and rats, expression of FcRn in intestinal epithelial cells is limited to the suckling period. We have recently observed, however, clear expression of FcRn in the adult human intestine, suggesting a function for FcRn in intestinal IgG transport beyond neonatal life in humans. We tested this hypothesis using the polarized human intestinal T84 cell line as a model epithelium. Immunocytochemical data show that FcRn is present in T84 cells in a punctate apical pattern similar to that found in human small intestinal enterocytes. Solute flux studies show that FcRn transports IgG across T84 monolayers by receptor-mediated transcytosis. Transport is bidirectional, specific for FcRn, and dependent upon endosomal acidification. These data define a novel bidirectional mechanism of IgG transport across epithelial barriers that predicts an important effect of FcRn on IgG function in immune surveillance and host defense at mucosal surfaces.
Figures
Figure 1
FcRn expression in normal adult human small intestine and human intestinal epithelial cell lines. Western blots of total cellular protein (13 μg protein per lane, a; 10 μg protein per lane, b) isolated from the indicated source using affinity-purified rabbit antisera raised against amino acids 112–125 (a) or amino acids 174–188 (b). (c) RT-PCR detection of FcγRI transcripts. Total RNA (2 μg) from T84 (lanes 3 and 4), MOLT-4 (lanes 5 and 6; negative control), and U937 (lanes 1 and 2; positive control) cell lines was incubated with an oligo-dT primer with (odd-numbered lanes) or without (even-numbered lanes) avian myeloblastosis virus–RT (AMV-RT), and a nested PCR was performed with primers specific for FcγRI cDNA (top) or for β-actin (bottom).
Figure 2
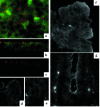
Immunolocalization of FcRn in polarized T84 monolayers (a–c) and normal adult human small intestinal mucosa (d–g). (a) Whole-mount T84 monolayers show a diffuse, punctate staining pattern. The Z0-1 image was captured slightly above the focal plane of FcRn. (b) FcRn staining of whole-mount T84 monolayers viewed as confocal vertical sections. (c) FcRn staining was absent in the presence of an isotype-matched, irrelevant antiserum. (f) Villous enterocytes of normal adult human small intestine show delicate linear staining in the region of the apical cytoplasmic membrane. (g) Crypt enterocytes show an apical and punctate staining pattern visible not only at the level of the apical cytoplasmic membrane, but also in the apical cytoplasm below the level of the apical membrane. (d and e) FcRn staining was absent in the presence of an irrelevant antiserum or with secondary antibody alone (not shown).
Figure 3
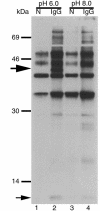
Functional expression of FcRn in polarized T84 cells. T84 cells grown on collagen-coated Transwells were cell surface–biotinylated and solubilized at either pH 6.0 or pH 8.0 lysates were incubated with Sepharose beads (N) or beads coupled to IgG (IgG). Immunoprecipitated proteins were analyzed by SDS-PAGE and avidin blot. A 12-kDa band consistent with the mobility of β2M (small arrow) and a 45-kDa band consistent with the mobility of human FcRn α chain (large arrow) were immunoprecipitated by IgG-coupled beads at pH 6.0 but not at pH 8.0.
Figure 4
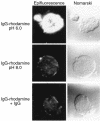
Binding of rhodamine-labeled IgG to T84 cell surfaces. T84 cells grown on glass coverslips were incubated at 4°C with rhodamine-labeled IgG at pH 6.0 or pH 8.0 in the presence or absence of excess competing nonlabeled IgG. Ligand binding was assessed by epifluorescence and Nomarski bright-field microscopy.
Figure 5
FcRn-dependent bidirectional transcytosis of IgG across T84 cell monolayers. (a) Transcytosis of IgG-biotin occurs at 37°C (lanes 1 and 3) but not at 4°C (lane 2). (b) Specificity of IgG transport for FcRn. T84 cells transport human IgG-biotin at 37°C (lane 3) but not at 4°C (lane 4). IgG heavy and light chains are indicated by the bars to the left of the IgG standard. In contrast, chicken IgY-biotin does not cross T84 cell monolayers at 37°C in either direction (lanes 2 and 5). IgY heavy and light chains are indicated by asterisks to the right of the IgY standard. (c) Quantitative ELISA of IgG and IgY transport at 37°C and 4°C.
Figure 6
Specificity of IgG transport and functional dependence on the vacuolar H+ ATPase. Where transport occured, the 25-kDa biotinylated light chain of IgG is shown. (a) Apical-to-basolateral transcytosis of IgG-biotin (60 nM) in the absence or presence of a 500-fold molar excess of nonlabeled chicken IgY (30 μM) or human IgG (30 μM) (lanes 4 and 5, respectively). Lanes 2 and 3 show incubation in the absence of competitive inhibitor at 37°C and 4°C as positive and negative controls, respectively. (b) Basolateral-to-apical transcytosis of IgG-biotin (60 nM) in the absence or presence of a 500-fold molar excess of nonlabeled chicken IgY (30 μM) (lane 3) or human IgG (30 μM) (lane 4) or excess fragment B of Staphylococcal protein A (0.1 mg/mL; lane 5). Lane 2 shows incubation at 37°C as a positive control. (c) T84 cells were incubated in the presence (lane 2) or absence (lanes 3 and 4) of bafilomycin A1 (0.1 μM), and basolaterally directed transport of IgG-biotin (60 nM) was measured after 1 hour of incubation in symmetrical HBSS+ buffered to pH 8.0.
Similar articles
- Receptor-mediated immunoglobulin G transport across mucosal barriers in adult life: functional expression of FcRn in the mammalian lung.
Spiekermann GM, Finn PW, Ward ES, Dumont J, Dickinson BL, Blumberg RS, Lencer WI. Spiekermann GM, et al. J Exp Med. 2002 Aug 5;196(3):303-10. doi: 10.1084/jem.20020400. J Exp Med. 2002. PMID: 12163559 Free PMC article. - Bidirectional transcytosis of IgG by the rat neonatal Fc receptor expressed in a rat kidney cell line: a system to study protein transport across epithelia.
McCarthy KM, Yoong Y, Simister NE. McCarthy KM, et al. J Cell Sci. 2000 Apr;113 ( Pt 7):1277-85. doi: 10.1242/jcs.113.7.1277. J Cell Sci. 2000. PMID: 10704378 - N-Glycan Moieties in Neonatal Fc Receptor Determine Steady-state Membrane Distribution and Directional Transport of IgG.
Kuo TT, de Muinck EJ, Claypool SM, Yoshida M, Nagaishi T, Aveson VG, Lencer WI, Blumberg RS. Kuo TT, et al. J Biol Chem. 2009 Mar 27;284(13):8292-300. doi: 10.1074/jbc.M805877200. Epub 2009 Jan 21. J Biol Chem. 2009. PMID: 19164298 Free PMC article. - New functions of the MHC class I-related Fc receptor, FcRn.
Simister NE, Jacobowitz Israel E, Ahouse JC, Story CM. Simister NE, et al. Biochem Soc Trans. 1997 May;25(2):481-6. doi: 10.1042/bst0250481. Biochem Soc Trans. 1997. PMID: 9191140 Review. - [Neonatal Fc receptor, key control of immunoglobulins biodistribution].
Magdelaine-Beuzelin C, Ohresser M, Watier H. Magdelaine-Beuzelin C, et al. Med Sci (Paris). 2009 Dec;25(12):1053-6. doi: 10.1051/medsci/200925121053. Med Sci (Paris). 2009. PMID: 20035678 Review. French.
Cited by
- Maternal immunity enhances systemic recall immune responses upon oral immunization of piglets with F4 fimbriae.
Nguyen UV, Melkebeek V, Devriendt B, Goetstouwers T, Van Poucke M, Peelman L, Goddeeris BM, Cox E. Nguyen UV, et al. Vet Res. 2015 Jun 23;46(1):72. doi: 10.1186/s13567-015-0210-3. Vet Res. 2015. PMID: 26100608 Free PMC article. - The Murine Neonatal Fc Receptor Is Required for Transport of Immunization-Induced C. difficile-Specific IgG to the Gut and Protection against Disease but Does Not Affect Disease Susceptibility.
Amadou Amani S, Lang GA, Ballard JD, Lang ML. Amadou Amani S, et al. Infect Immun. 2021 Sep 16;89(10):e0027421. doi: 10.1128/IAI.00274-21. Epub 2021 Jun 7. Infect Immun. 2021. PMID: 34097471 Free PMC article. - Ganglioside GM1-mediated transcytosis of cholera toxin bypasses the retrograde pathway and depends on the structure of the ceramide domain.
Saslowsky DE, Te Welscher YM, Chinnapen DJ, Wagner JS, Wan J, Kern E, Lencer WI. Saslowsky DE, et al. J Biol Chem. 2013 Sep 6;288(36):25804-25809. doi: 10.1074/jbc.M113.474957. Epub 2013 Jul 24. J Biol Chem. 2013. PMID: 23884419 Free PMC article. - The neonatal Fc receptor, FcRn, as a target for drug delivery and therapy.
Sockolosky JT, Szoka FC. Sockolosky JT, et al. Adv Drug Deliv Rev. 2015 Aug 30;91:109-24. doi: 10.1016/j.addr.2015.02.005. Epub 2015 Feb 19. Adv Drug Deliv Rev. 2015. PMID: 25703189 Free PMC article. Review. - Clinical ramifications of the MHC family Fc receptor FcRn.
Roopenian DC, Sun VZ. Roopenian DC, et al. J Clin Immunol. 2010 Nov;30(6):790-7. doi: 10.1007/s10875-010-9458-6. Epub 2010 Sep 17. J Clin Immunol. 2010. PMID: 20848168 Free PMC article. Review.
References
- Brambell FW. The transmission of immunity from mother to young and the catabolism of immunoglobulins. Lancet. 1966;2:1087–1093. - PubMed
- Simister NE, Rees AR. Isolation and characterization of an Fc receptor from neonatal rat small intestine. Eur J Immunol. 1985;15:733–738. - PubMed
- Simister NE, Story CM, Chen HL, Hunt JS. An IgG-transporting Fc receptor expressed in the syncytiotrophoblast of human placenta. Eur J Immunol. 1996;26:1527–1531. - PubMed
- Simister NE, Mostov KE. Cloning and expression of the neonatal rat intestinal Fc receptor, a major histocompatibility complex class I antigen homolog. Cold Spring Harb Symp Quant Biol. 1989;54:571–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 DK044319/DK/NIDDK NIH HHS/United States
- R01 DK053056/DK/NIDDK NIH HHS/United States
- R01 HD027691/HD/NICHD NIH HHS/United States
- R01 DK048106/DK/NIDDK NIH HHS/United States
- HD-27691/HD/NICHD NIH HHS/United States
- R01 AI053056/AI/NIAID NIH HHS/United States
- P30 DK034854/DK/NIDDK NIH HHS/United States
- DK/AI-53056/DK/NIDDK NIH HHS/United States
- R56 DK053056/DK/NIDDK NIH HHS/United States
- DK-44139/DK/NIDDK NIH HHS/United States
- R01 DK044319/DK/NIDDK NIH HHS/United States
- R37 DK048106/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials