Rab5 induces Rac-independent lamellipodia formation and cell migration - PubMed (original) (raw)
Rab5 induces Rac-independent lamellipodia formation and cell migration
M Spaargaren et al. Mol Biol Cell. 1999 Oct.
Free PMC article
Abstract
Rab5 is a regulatory GTPase of vesicle docking and fusion that is involved in receptor-mediated endocytosis and pinocytosis. Introduction of active Rab5 in cells stimulates the rate of endocytosis and vesicle fusion, resulting in the formation of large endocytic vesicles, whereas dominant negative Rab5 inhibits vesicle fusion. Here we show that introduction of active Rab5 in fibroblasts also induced reorganization of the actin cytoskeleton but not of microtubule filaments, resulting in prominent lamellipodia formation. The Rab5-induced lamellipodia formation did not require activation of PI3-K or the GTPases Ras, Rac, Cdc42, or Rho, which are all strongly implicated in cytoskeletal reorganization. Furthermore, lamellipodia formation by insulin, Ras, or Rac was not affected by expression of dominant negative Rab5. In addition, cells expressing active Rab5 displayed a dramatic stimulation of cell migration, with the lamellipodia serving as the leading edge. Both lamellipodia formation and cell migration were dependent on actin polymerization but not on microtubules. These results demonstrate that Rab5 induces lamellipodia formation and cell migration and that the Rab5-induced lamellipodia formation occurs by a novel mechanism independent of, and distinct from, PI3-K, Ras, or Rho-family GTPases. Thus, Rab5 can control not only endocytosis but also actin cytoskeleton reorganization and cell migration, which provides strong support for an intricate relationship between these processes.
Figures
Figure 1
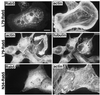
L79-Rab5, but not N34-Rab5, induces the formation of large endosomes and lamellipodia. Shown is immunofluorescence microscopy of A14 fibroblasts grown on coverslips and expressing either L79-Rab5 or N34-Rab5 as indicated. Rab5 expression and localization were visualized by anti-HA × GαM-Cy3 staining, the actin cytoskeleton was visualized by phalloidin-FITC, and microtubules were visualized by anti-tubulin × GαM-Cy3 (as indicated). The same cells are shown in the left and right panels. Bar, 30 μm.
Figure 2
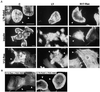
Rab5-induced lamellipodia formation is PI3-K– and Rac-independent. Shown is immunofluorescence microscopy of the actin cytoskeleton of A14 cells (A) transfected with either L79-Rab5 or V12-Ras or stimulated with insulin (as indicated per row) and (co)transfected with control plasmid or N17-Rac or treated with LY 294002 (as indicated per column), or (B) cotransfected with V12-Ras and PAK-RBD or L79-Rab5 and PAK-RBD, as indicated. Arrowheads indicate the Cy3-positive cells stained for the expression of L79-Rab5, V12-Ras, or in case of their cotransfection with L79-Rab5, N17-Rac, or PAK-RBD. Only the actin staining as detected by phalloidin-FITC is shown. Bar, 30 μm. The scale bar in the top left picture applies to all others, unless a different size scale bar is shown.
Figure 3
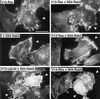
Rab5-induced lamellipodia formation is Ras-, Cdc42-, and Rho-independent. Immunofluorescence microscopy of the actin cytoskeleton of A14 cells cotransfected with L79-Rab5 and N17-Ras, N17-Cdc42, or N19-Rho is shown. Arrowheads indicate the Cy3-positive cells stained for expression of the dominant negative GTPases. Only the actin staining as detected by phalloidin-FITC is shown. Bar, 30 μm.
Figure 4
Insulin-, Ras-, and Rac-induced lamellipodia formation is Rab5-independent. Shown is immunofluorescence microscopy of the actin cytoskeleton of A14 cells transfected with V12-Rac alone, or cotransfected with V12-Rac, V12-Ras, V12-Cdc42, or V14-Rho and N34-Rab5, or transfected with N34-Rab5 alone and stimulated for 5 min with insulin (as indicated). Arrowheads indicate the Cy3-positive cells stained for expression of either V12-Rac or, in the case of cotransfection, N34-Rab5. Only the actin staining as detected by phalloidin-FITC is shown. Bar, 30 μm.
Figure 5
Rab5 induces cell migration that is actin polymerization-dependent but microtubule-independent. Shown is time-lapse video microscopy of the migration of A14 cells that were (A) transfected with L79-Rab5 (please note that the cell only starts to migrate after polarizing its lamellipodium), (B) L79-Rab5–transfected and treated at t = 58 min with either 1 μM cytochalasin D (please note that only the cell with the polarized lamellipodium in the bottom right corner shows migration between 0 and 20 min, until its lamellipodium depolarizes), and (C) L79-Rab5–transfected and treated at t = 0 min with 10 μg/ml nocodazole. The inset (D) shows the actin cytoskeleton and confirms depolymerization of microtubules (compare with Figure 1) of cells, including a cell displaying a lamellipodium as a consequence of L79-Rab5 transfection, after treatment with 10 μg/ml nocodazole for 30 min. Bar, 30 μm.
Figure 6
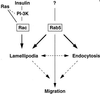
Distinct mechanisms for Rac- and Rab5-induced biological responses. The reorganization of the actin cytoskeleton is induced independently by Rac and Rab5. In addition, the Rab5-induced lamellipodia formation and endocytosis may be regulated by distinct mechanisms as well; however, there is a clear relationship between these two events, suggesting that Rab5 may control cytoskeletal reorganization to provide support and direction for the endocytic events. Furthermore, several models have been proposed in which the driving force for cell migration is either a polarized actin polymerization cycle or a polarized endocytic/exocytic cycle. We propose that the dramatic effect of Rab5 on cell migration, which was not observed with Ras or Rac, may be the consequence of the combined action of Rab5-induced actin cytoskeleton reorganization and endocytosis. See DISCUSSION and CONCLUSION for further details.
Similar articles
- Role of actin polymerization and adhesion to extracellular matrix in Rac- and Rho-induced cytoskeletal reorganization.
Machesky LM, Hall A. Machesky LM, et al. J Cell Biol. 1997 Aug 25;138(4):913-26. doi: 10.1083/jcb.138.4.913. J Cell Biol. 1997. PMID: 9265656 Free PMC article. - Adenovirus endocytosis requires actin cytoskeleton reorganization mediated by Rho family GTPases.
Li E, Stupack D, Bokoch GM, Nemerow GR. Li E, et al. J Virol. 1998 Nov;72(11):8806-12. doi: 10.1128/JVI.72.11.8806-8812.1998. J Virol. 1998. PMID: 9765425 Free PMC article. - Rab5 is a signalling GTPase involved in actin remodelling by receptor tyrosine kinases.
Lanzetti L, Palamidessi A, Areces L, Scita G, Di Fiore PP. Lanzetti L, et al. Nature. 2004 May 20;429(6989):309-14. doi: 10.1038/nature02542. Nature. 2004. PMID: 15152255 - Ras-related GTPases and the cytoskeleton.
Hall A. Hall A. Mol Biol Cell. 1992 May;3(5):475-9. doi: 10.1091/mbc.3.5.475. Mol Biol Cell. 1992. PMID: 1611153 Free PMC article. Review. - On the role of Rab5 in cell migration.
Mendoza P, Díaz J, Torres VA. Mendoza P, et al. Curr Mol Med. 2014 Feb;14(2):235-45. doi: 10.2174/1566524014666140128111347. Curr Mol Med. 2014. PMID: 24467205 Review.
Cited by
- Rab5 activation promotes focal adhesion disassembly, migration and invasiveness in tumor cells.
Mendoza P, Ortiz R, Díaz J, Quest AF, Leyton L, Stupack D, Torres VA. Mendoza P, et al. J Cell Sci. 2013 Sep 1;126(Pt 17):3835-47. doi: 10.1242/jcs.119727. Epub 2013 Jun 26. J Cell Sci. 2013. PMID: 23813952 Free PMC article. - Rab'ing tumor cell migration and invasion: focal adhesion disassembly driven by Rab5.
Torres VA. Torres VA. Cell Adh Migr. 2014;8(2):84-7. doi: 10.4161/cam.28510. Cell Adh Migr. 2014. PMID: 24727246 Free PMC article. - Mechanism by which Rab5 promotes regeneration and functional recovery of zebrafish Mauthner axons.
Cui J, Shen Y, Song Z, Fan D, Hu B. Cui J, et al. Neural Regen Res. 2025 Jun 1;20(6):1816-1824. doi: 10.4103/NRR.NRR-D-23-00529. Epub 2024 Apr 3. Neural Regen Res. 2025. PMID: 39104118 Free PMC article. - Endocytic and recycling endosomes modulate cell shape changes and tissue behaviour during morphogenesis in Drosophila.
Mateus AM, Gorfinkiel N, Schamberg S, Martinez Arias A. Mateus AM, et al. PLoS One. 2011 Apr 14;6(4):e18729. doi: 10.1371/journal.pone.0018729. PLoS One. 2011. PMID: 21533196 Free PMC article. - Klf5 controls bone marrow homing of stem cells and progenitors through Rab5-mediated β1/β2-integrin trafficking.
Taniguchi Ishikawa E, Chang KH, Nayak R, Olsson HA, Ficker AM, Dunn SK, Madhu MN, Sengupta A, Whitsett JA, Grimes HL, Cancelas JA. Taniguchi Ishikawa E, et al. Nat Commun. 2013;4:1660. doi: 10.1038/ncomms2645. Nat Commun. 2013. PMID: 23552075 Free PMC article.
References
- Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. - PubMed
- Bos JL. Ras-like GTPases. Biochim Biophys Acta. 1997;1333:M19–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous