Nascent polypeptide-associated complex stimulates protein import into yeast mitochondria - PubMed (original) (raw)
Nascent polypeptide-associated complex stimulates protein import into yeast mitochondria
U Fünfschilling et al. Mol Biol Cell. 1999 Oct.
Free PMC article
Abstract
To identify yeast cytosolic proteins that mediate targeting of precursor proteins to mitochondria, we developed an in vitro import system consisting of purified yeast mitochondria and a radiolabeled mitochondrial precursor protein whose C terminus was still attached to the ribosome. In this system, the N terminus of the nascent chain was translocated across both mitochondrial membranes, generating a translocation intermediate spanning both membranes. The nascent chain could then be completely chased into the mitochondrial matrix after release from the ribosome. Generation of this import intermediate was dependent on a mitochondrial membrane potential, mitochondrial surface proteins, and was stimulated by proteins that could be released from the ribosomes by high salt. The major salt-released stimulatory factor was yeast nascent polypeptide-associated complex (NAC). Purified NAC fully restored import of salt-washed ribosome-bound nascent chains by enhancing productive binding of the chains to mitochondria. We propose that ribosome-associated NAC facilitates recognition of nascent precursor chains by the mitochondrial import machinery.
Figures
Figure 1
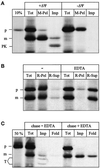
Ribosome-associated nascent chains can generate a mitochondrial import intermediate spanning both membranes. (A) Generation of the import intermediate. RNCs carrying the radiolabeled Mdh1p precursor were isolated and incubated with yeast mitochondria as described in MATERIALS AND METHODS for 20 min at 20°C in the absence (+ΔΨ) or presence (−ΔΨ) of 1 μg/ml valinomycin. Equal aliquots were precipitated with TCA (Tot), centrifuged for 3 min at 14,000 × g to recover the mitochondria (M-Pel), or treated with 100 μg/μl proteinase K for 15 min on ice, followed by inactivation of the protease with 1 mM PMSF, reisolation of the mitochondria, and precipitation with TCA (Imp). All samples were analyzed by SDS-PAGE and fluorography. 10%, 10% of the RNCs added per lane. (B) The intermediate is ribosome attached. Import was performed as in A and stopped by transfer to 0°C. The sample was then split into two equal aliquots. The mitochondria in both aliquots were solubilized in 1% Triton X-100 either in the presence (EDTA) or absence (−) of 10 mM EDTA as described in MATERIALS AND METHODS. One-half of each aliquot was precipitated with TCA (Tot); the other half was separated into a ribosomal pellet (R-Pel) and a supernatant (R-Sup). All samples were analyzed by SDS-PAGE and fluorography. (C) Chase of the intermediate. Import was performed as in A, and further generation of the import intermediate was stopped after 20 min by the addition of 1 μg/ml valinomycin. The reaction was split into two equal aliquots. Onealiquot was left untreated at 20°C (chase − EDTA); the other aliquot was treated with 10 mM EDTA for 10 min at 20°C, followed by the addition of 10 mM MgAcetate (chase + EDTA). One-third of each aliquot was precipitated with TCA (Tot); the remaining two-thirds were treated with 100 μg/ml trypsin for 30 min on ice. After inactivation of the protease with 200 μg/ml soy bean trypsin inhibitor and reisolation of the mitochondria, the trypsin-treated mitochondria were subdivided in two equal samples. The first sample was precipitated with TCA (Imp), and the second sample was treated with 1% Triton X-100 plus 100 μg/ml proteinase K for 15 min on ice. After inactivation of the protease with 1 mM PMSF, the second sample was also precipitated with TCA (Fold). All samples were analyzed by SDS-PAGE and fluorography. 50%, 50% of the RNCs added per lane; p, Mdh1p precursor; m, mature Mdh1p; PK, proteinase K-protected fragment; T, trypsin-protected fragments.
Figure 2
Salt washing of RNCs reversibly reduces import of the attached nascent chains. Untreated RNCs, salt-washed RNCs, and a ribosomal salt wash fluid were prepared as described in MATERIALS AND METHODS. Salt wash fluid was prepared from an amount of ribosomes corresponding to the amount of ribosomes present as RNCs in the import reaction. Import reactions were performed with untreated RNCs, salt-washed RNCs, and salt-washed RNCs mixed with salt wash fluid (+ salt-wash fluid) as described in MATERIALS AND METHODS. After the indicated times, equal samples were precipitated with TCA and analyzed by SDS-PAGE and fluorography. p, Mdh1p precursor; m, mature Mdh1p.
Figure 3
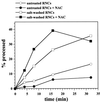
Immunodepletion of NAC from salt wash fluid depletes most but not all import stimulatory activity. (A) Immunodepletion of salt wash fluid. Equal aliquots of salt wash fluid (see Figure 2) were either left untreated (tot), mock depleted with preimmuneserum (mock-depleted), or immunodepleted with immuneserum against αNAC (αNAC-depleted). The treated aliquots were separated into supernatant (sup) and beads (pel), and all samples were analyzed for αNAC by SDS-PAGE and immunoblotting. (B) Import stimulation by salt wash fluid. Import reactions were performed with salt-washed RNCs without addition (−), after addition of mock-depleted salt wash fluid (mock-depleted), or after addition of αNAC-depleted salt wash fluid (αNAC-depleted) as described in MATERIALS AND METHODS. After the indicated times, equal samples were centrifuged for 3 min at 14,000 × g to recover the mitochondria. All samples were analyzed by SDS-PAGE and fluorography and quantified by densitometry. % import, percentage of mature Mdh1p given as fraction of initially added Mdh1p; 20%, 20% of the RNCs added per lane; p, Mdh1p precursor; m, mature Mdh1p.
Figure 4
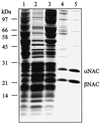
Purification of NAC from yeast cytosol. Yeast NAC was purified as described in MATERIALS AND METHODS, and the following fractions were analyzed by SDS-PAGE and staining with Coomassie blue: lane 1, cytosol; lane 2, ribosomal pellet; lane 3, salt wash fluid; lane 4, pooled fractions after ResourceQ chromatography; lane 5, pooled fractions after MonoQ chromatography. Lanes 1–3 contain 100 μg of protein; lanes 4 and 5 contain 10 μg of protein. Molecular mass standards are indicated on the left.
Figure 5
Purified NAC stimulates import of ribosome-associated nascent chains into mitochondria. Import reactions were performed with salt-washed RNCs in the presence of the indicated amounts of purified NAC in a total volume of 100 μl as described in MATERIALS AND METHODS. After 15 min of import, all samples were centrifuged for 3 min at 14,000 × g to recover the mitochondria and were analyzed by SDS-PAGE and fluorography. The resulting bands on the fluorogram were quantified by densitometry, and the percentage of processed chains was determined, with the amount of initially added Mdh1p set to 100% (% import). 10%, 10% of the RNCs added per lane; p, Mdh1p precursor; m, mature Mdh1p.
Figure 6
Salt washing of RNCs increases the accessibility of the attached nascent chains to MPP in vitro. Untreated and salt-washed RNCs were incubated in import buffer with 0.5 μM purified MPP in the presence or absence of 40 μg/ml purified NAC at 20°C for the indicated times. Cleavage of the Mdh1p precursor was analyzed by SDS-PAGE and fluorography. The percentage of cleaved chains was determined by densitometry of the fluorogram, with the amount of initially added Mdh1p set to 100%.
Figure 7
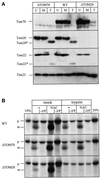
Stimulation of import by NAC depends on mitochondrial surface proteins. (A) Trypsin treatment of mitochondria. Three aliquots of crude mitochondria from yeast strains YVH1 (ΔTOM70), JKR101 (WT), or YVH1 (ΔTOM20) each were treated as follows. The first aliquot was left untreated on ice (U); the second aliquot was treated with 100 μg/ml trypsin for 5min on ice, followed by inactivation of trypsin with 200 μg/ml soybean trypsin inhibitor for 5 min and reisolation of the mitochondria (T); and the third aliquot was mock-treated with 100 μg/ml trypsin that had been inactivated by preincubation with 200 μg/ml trypsin inhibitor for 5 min on ice followed by reisolation of the mitochondria (M). Equal amounts of mitochondrial protein (75 μg) were analyzed by SDS-PAGE and immunoblotting for Tom70p, Tom20p, Tom22p, and Tim23p as indicated on the side. Tom20* and Tom22* denote a trypsin-generated fragment of Tom20p and Tom22p, respectively. (B) Mitochondrial surface proteins are required for import stimulation by NAC. Imports were performed with the same trypsin-treated (trypsin) or mock-treated (mock) mitochondria as shown in A with salt-treated RNCs in the presence (NAC) or absence (−) of 10 μg/ml NAC. The strain is indicated on the left. After 7.5 min, mitochondria were recovered by centrifugation for 3 min at 14,000 × g, and analyzed by SDS-PAGE and fluorography. 10 and 20%, 10 and 20% of the RNCs added per lane, respectivey; −ΔΨ, import in the presence of 1 μg/ml valinomycin and 25 μM carbonyl cyanide _p-_trifluoromethoxyphenyl-hydrazone; p, Mdh1p precursor; m, mature Mdh1p.
Figure 8
NAC promotes productive binding of RNCs to mitochondria. Salt-washed RNCs were incubated with 0.15 mg/ml mitochondria in import buffer for 5 min on ice in the presence (NAC) or absence (−) of 11 μg/ml NAC. The mitochondria were reisolated, resuspended in import buffer, and divided in two aliquots. One aliquot was precipitated with TCA (bound). The other aliquot was supplemented with 2 mM ATP, NADH, and KPi and shifted to 20°C. After the indicated chase times, samples were withdrawn and precipitated with TCA and analyzed by SDS-PAGE and fluorography. −ΔΨ, chase for 2 min in the presence of 1 μg/ml valinomycin; 2.5%, 2.5% of the RNCs added per lane; p, Mdh1p precursor; m, mature Mdh1p.
Figure 9
Proposed role of NAC in mitochondrial protein import. From left to right: untreated RNCs contain several salt-extractable proteins, including NAC (black ellipse), which keep the nascent chain import competent (depicted by the helical conformation of the positively charged presequence). Salt washing removes these proteins and exposes the nascent chain to purified MPP and lowers its import competence. Readdition of purified NAC does not shield the nascent chain against purified MPP but restores import competence of the nascent chain by presenting it in a conformation recognized by mitochondrial surface receptors.
Similar articles
- OM14 is a mitochondrial receptor for cytosolic ribosomes that supports co-translational import into mitochondria.
Lesnik C, Cohen Y, Atir-Lande A, Schuldiner M, Arava Y. Lesnik C, et al. Nat Commun. 2014 Dec 9;5:5711. doi: 10.1038/ncomms6711. Nat Commun. 2014. PMID: 25487825 Free PMC article. - αβ'-NAC cooperates with Sam37 to mediate early stages of mitochondrial protein import.
Ponce-Rojas JC, Avendaño-Monsalve MC, Yañez-Falcón AR, Jaimes-Miranda F, Garay E, Torres-Quiroz F, DeLuna A, Funes S. Ponce-Rojas JC, et al. FEBS J. 2017 Mar;284(5):814-830. doi: 10.1111/febs.14024. Epub 2017 Feb 12. FEBS J. 2017. PMID: 28109174 - The intrinsic ability of ribosomes to bind to endoplasmic reticulum membranes is regulated by signal recognition particle and nascent-polypeptide-associated complex.
Lauring B, Kreibich G, Weidmann M. Lauring B, et al. Proc Natl Acad Sci U S A. 1995 Oct 10;92(21):9435-9. doi: 10.1073/pnas.92.21.9435. Proc Natl Acad Sci U S A. 1995. PMID: 7568149 Free PMC article. - Membrane protein import in yeast mitochondria.
Tokatlidis K, Vial S, Luciano P, Vergnolle M, Clémence S. Tokatlidis K, et al. Biochem Soc Trans. 2000;28(4):495-9. Biochem Soc Trans. 2000. PMID: 10961947 Review. - Mitochondrial protein import.
Geli V, Glick B. Geli V, et al. J Bioenerg Biomembr. 1990 Dec;22(6):725-51. doi: 10.1007/BF00786928. J Bioenerg Biomembr. 1990. PMID: 2092036 Review.
Cited by
- RNA trafficking in plant cells: targeting of cytosolic mRNAs to the mitochondrial surface.
Michaud M, Maréchal-Drouard L, Duchêne AM. Michaud M, et al. Plant Mol Biol. 2010 Aug;73(6):697-704. doi: 10.1007/s11103-010-9650-3. Epub 2010 May 27. Plant Mol Biol. 2010. PMID: 20506035 - Defining the specificity of cotranslationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes.
del Alamo M, Hogan DJ, Pechmann S, Albanese V, Brown PO, Frydman J. del Alamo M, et al. PLoS Biol. 2011 Jul;9(7):e1001100. doi: 10.1371/journal.pbio.1001100. Epub 2011 Jul 12. PLoS Biol. 2011. PMID: 21765803 Free PMC article. - Import-associated translational inhibition: novel in vivo evidence for cotranslational protein import into Dictyostelium discoideum mitochondria.
Ahmed AU, Beech PL, Lay ST, Gilson PR, Fisher PR. Ahmed AU, et al. Eukaryot Cell. 2006 Aug;5(8):1314-27. doi: 10.1128/EC.00386-05. Eukaryot Cell. 2006. PMID: 16896215 Free PMC article. - NAC regulates metabolism and cell fate in intestinal stem cells.
Ramalho S, Alkan F, Prekovic S, Jastrzebski K, Barberà EP, Hoekman L, Altelaar M, de Heus C, Liv N, Rodríguez-Colman MJ, Yilmaz M, van der Kammen R, Fedry J, de Gooijer MC, Suijkerbuijk SJE, Faller WJ, Silva J. Ramalho S, et al. Sci Adv. 2025 Jan 10;11(2):eadn9750. doi: 10.1126/sciadv.adn9750. Epub 2025 Jan 8. Sci Adv. 2025. PMID: 39772672 Free PMC article. - Prion-dependent switching between respiratory competence and deficiency in the yeast nam9-1 mutant.
Chacinska A, Boguta M, Krzewska J, Rospert S. Chacinska A, et al. Mol Cell Biol. 2000 Oct;20(19):7220-9. doi: 10.1128/MCB.20.19.7220-7229.2000. Mol Cell Biol. 2000. PMID: 10982839 Free PMC article.
References
- Ades IZ, Butow RA. The products of mitochondria-bound cytoplasmic polysomes in yeast. J Biol Chem. 1980;255:9918–9924. - PubMed
- Branda SS, Isaya G. Prediction and identification of new natural substrates of the yeast mitochondrial intermediate peptidase. J Biol Chem. 1995;270:27366–27373. - PubMed
- Brix J, Rüdiger S, Bukau B, Schneider-Mergener J, Pfanner N. Distribution of binding sequences for the mitochondrial import receptors Tom20, Tom22, and Tom70 in a presequence-carrying preprotein and a noncleavable preprotein. J Biol Chem. 1999;274:16522–16530. - PubMed
- Caplan AJ, Cyr DM, Douglas MG. YDJ1p facilitates polypeptide translocation across different intracellular membranes by a conserved mechanism. Cell. 1992;71:1143–1155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases