A role for cadherin-5 in regulation of vascular endothelial growth factor receptor 2 activity in endothelial cells - PubMed (original) (raw)
A role for cadherin-5 in regulation of vascular endothelial growth factor receptor 2 activity in endothelial cells
N Rahimi et al. Mol Biol Cell. 1999 Oct.
Free PMC article
Abstract
FLK-1/vascular endothelial growth factor receptor 2 (VEGFR-2) is one of the receptors for VEGF. In this study we examined the effect of cell density on activation of VEGFR-2. VEGF induces only very slight tyrosine phosphorylation of VEGFR-2 in confluent (95-100% confluent) pig aortic endothelial (PAE) cells. In contrast, robust VEGF-dependent tyrosine phosphorylation of VEGFR-2 was observed in cells plated in sparse culture conditions (60-65% confluent). A similar cell density-dependent phenomenon was observed in different endothelial cells but not in NIH-3T3 fibroblast cells expressing VEGFR-2. Stimulating cells with high concentrations of VEGF or replacing the extracellular domain of VEGFR-2 with that of the colony-stimulating factor 1 receptor did not alleviate the sensitivity of VEGFR-2 to cell density, indicating that the confluent cells were probably not secreting an antagonist to VEGF. Furthermore, in PAE cells, ectopically introduced platelet-derived growth factor alpha receptor could be activated at both high and low cell density conditions, indicating that the density effect was not universal for all receptor tyrosine kinases expressed in endothelial cells. In addition to lowering the density of cells, removing divalent cations from the medium of confluent cells potentiated VEGFR-2 phosphorylation in response to VEGF. These findings suggested that cell-cell contact may be playing a role in regulating the activation of VEGFR-2. To this end, pretreatment of confluent PAE cells with a neutralizing anti-cadherin-5 antibody potentiated the response of VEGFR-2 to VEGF. Our data demonstrate that endothelial cell density plays a critical role in regulating VEGFR-2 activity, and that the underlying mechanism appears to involve cadherin-5.
Figures
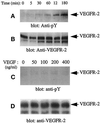
Figure 1
Effect of endothelial cell density on activation of VEGFR-2. An equal number of PAE cells overexpressing VEGFR-2 or AEC cells endogenously expressing VEGFR-2 were cultured in 10-cm (dense condition) or 15-cm (sparse condition) tissue culture plates, serum starved overnight, and stimulated with VEGF (100 ng/ml) for 5 min. Cells were lysed and immunoprecipitated with an anti-VEGFR-2 antibody and immunoblotted with an anti-phosphotyrosine (pY) antibody (A and D) or subjected to an in vitro kinase assay (C). To determine the protein levels in each lane, the same membranes were reprobed with an anti-VEGFR-2 antibody (B and E).
Figure 2
Kinetics of tyrosine phosphorylation of VEGFR-2 in confluent PAE cells. Serum-starved confluent PAE cells overexpressing VEGFR-2 were stimulated with VEGF (100 ng/ml) for the indicated times (A and B) or with the increasing concentrations of VEGF (C and D). Cells were lysed and immunoprecipitated with an anti-VEGFR-2 antibody. The immunoprecipitated proteins were collected, resolved on SDS-PAGE, transferred to an Immobilon membrane, and immunoblotted with an anti-phosphotyrosine (pY) antibody (A and C). To determine the protein levels in each lane, the same membrane was reprobed with an anti-VEGFR-2 antibody (B and D).
Figure 3
VEGFR-2 activation is not affected by cell density when expressed in NIH-3T3 cells. NIH-3T3 cells expressing VEGFR-2 were cultured in the confluent or sparse condition as described in the text, serum-starved overnight, and then stimulated with VEGF for 5 min. Cells were lysed and immunoprecipitated with an anti-VEGFR-2 antibody and immunoblotted with an anti-phosphotyrosine (pY) antibody (A). The same membrane was reprobed with an anti-VEGFR2 antibody (B).
Figure 4
PAE cell density does not alter PDGFR-α activation. PAE cells expressing PDGFR-α were cultured in the confluent or sparse condition, serum starved overnight, and then stimulated with PDGF-AA (40 ng/ml) for 5 min. Cells were lysed and immunoprecipitated with an PDGFR-α antibody and immunoblotted with an anti-phosphotyrosine (pY) antibody (A). The same membrane was reprobed with PDGFR-α antibody (B).
Figure 5
Replacement of the extracellular domain of VEGFR-2 with the extracellular domain of human CSF receptor-1 does not alter the behavior of VEGFR-2 when expressed in PAE cells. PAE cells expressing an empty vector (X2) or chimeric VEGFR-2 (CK) were cultured in the confluent or sparse condition, serum starved overnight, and stimulated with CSF (40 ng/ml) for 5 min. The cells were lysed and immunoprecipitated with an anti-VEGFR-2 antibody and imunoblotted with an anti-phosphotyrosine (pY) antibody (A). The same membrane was reprobed with an anti-VEGFR-2 antibody (B).
Figure 6
Pretreatment of confluent PAE cells with EGTA augments VEGF-induced tyrosine phosphorylation of VEGFR-2. Serum-starved confluent PAE cells overexpressing VEGFR-2 were treated with EGTA for the indicated periods and stimulated with VEGF for 5 min. Cells were lysed and immunoprecipitated with an anti-VEGFR-2 antibody. The immunoprecipitated proteins were collected, resolved on SDS-PAGE, and subjected to immunoblotting with an anti-phosphotyrosine (pY) antibody (A). The same membranes were reprobed with an anti-VEGFR-2 antibody (B).
Figure 7
Neutralizing anticadherin-5 antibody augments VEGF-induced tyrosine phosphorylation of VEGFR-2. Serum-starved confluent PAE cells overexpressing VEGFR-2 were treated with 20 μg/ml normal mouse antibody (NM IgG) or with 1 or 20 μg/ml anti-cadherin-5 antibody for 4 h. Cells were then stimulated with VEGF for 5 min. The cells were lysed and immunoprecipitated with an anti-VEGFR-2 antibody and probed with an anti-phosphotyrosine (pY) antibody (A). The same membranes was reprobed with an anti-VEGFR-2 antibody (B).
Similar articles
- The role of VEGF receptors in angiogenesis; complex partnerships.
Cébe-Suarez S, Zehnder-Fjällman A, Ballmer-Hofer K. Cébe-Suarez S, et al. Cell Mol Life Sci. 2006 Mar;63(5):601-15. doi: 10.1007/s00018-005-5426-3. Cell Mol Life Sci. 2006. PMID: 16465447 Free PMC article. Review. - Contact inhibition of VEGF-induced proliferation requires vascular endothelial cadherin, beta-catenin, and the phosphatase DEP-1/CD148.
Grazia Lampugnani M, Zanetti A, Corada M, Takahashi T, Balconi G, Breviario F, Orsenigo F, Cattelino A, Kemler R, Daniel TO, Dejana E. Grazia Lampugnani M, et al. J Cell Biol. 2003 May 26;161(4):793-804. doi: 10.1083/jcb.200209019. J Cell Biol. 2003. PMID: 12771128 Free PMC article. - Role of alphavbeta3 integrin in the activation of vascular endothelial growth factor receptor-2.
Soldi R, Mitola S, Strasly M, Defilippi P, Tarone G, Bussolino F. Soldi R, et al. EMBO J. 1999 Feb 15;18(4):882-92. doi: 10.1093/emboj/18.4.882. EMBO J. 1999. PMID: 10022831 Free PMC article. - Expression of vascular endothelial growth factor receptors in smooth muscle cells.
Ishida A, Murray J, Saito Y, Kanthou C, Benzakour O, Shibuya M, Wijelath ES. Ishida A, et al. J Cell Physiol. 2001 Sep;188(3):359-68. doi: 10.1002/jcp.1121. J Cell Physiol. 2001. PMID: 11473363 - Signal transduction induced in endothelial cells by growth factor receptors involved in angiogenesis.
Hofer E, Schweighofer B. Hofer E, et al. Thromb Haemost. 2007 Mar;97(3):355-63. Thromb Haemost. 2007. PMID: 17334501 Free PMC article. Review.
Cited by
- The role of VEGF receptors in angiogenesis; complex partnerships.
Cébe-Suarez S, Zehnder-Fjällman A, Ballmer-Hofer K. Cébe-Suarez S, et al. Cell Mol Life Sci. 2006 Mar;63(5):601-15. doi: 10.1007/s00018-005-5426-3. Cell Mol Life Sci. 2006. PMID: 16465447 Free PMC article. Review. - Axl is essential for VEGF-A-dependent activation of PI3K/Akt.
Ruan GX, Kazlauskas A. Ruan GX, et al. EMBO J. 2012 Apr 4;31(7):1692-703. doi: 10.1038/emboj.2012.21. Epub 2012 Feb 10. EMBO J. 2012. PMID: 22327215 Free PMC article. - Association of Csk to VE-cadherin and inhibition of cell proliferation.
Baumeister U, Funke R, Ebnet K, Vorschmitt H, Koch S, Vestweber D. Baumeister U, et al. EMBO J. 2005 May 4;24(9):1686-95. doi: 10.1038/sj.emboj.7600647. Epub 2005 Apr 7. EMBO J. 2005. PMID: 15861137 Free PMC article. - Signaling from the adherens junction.
McEwen AE, Escobar DE, Gottardi CJ. McEwen AE, et al. Subcell Biochem. 2012;60:171-96. doi: 10.1007/978-94-007-4186-7_8. Subcell Biochem. 2012. PMID: 22674072 Free PMC article. Review. - Decreased interleukin-20 level in patients with systemic sclerosis: are they related with angiogenesis?
Aydoğdu E, Pamuk ÖN, Dönmez S, Pamuk GE. Aydoğdu E, et al. Clin Rheumatol. 2013 Nov;32(11):1599-603. doi: 10.1007/s10067-013-2317-0. Epub 2013 Jun 29. Clin Rheumatol. 2013. PMID: 23812620
References
- Augustin HG, Kozian DH, Johnson RC. Differentiation of endothelial cells: analysis of the constitutive and activated endothelial cell phenotypes. Bioessays. 1994;12:901–906. - PubMed
- Breier G, Breviario F, Caveda L, Berthier R, Schnurch H, Gotsch U, Vestweber D, Risau W, Dejana E. Molecular cloning and expression of murine vascular endothelial-cadherin in early stage development of cardiovascular system. Blood. 1996;87:630–641. - PubMed
- Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–571. - PubMed
- Cines DB, et al. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials