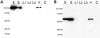The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex - PubMed (original) (raw)
The Saccharomyces cerevisiae homologue of human Wiskott-Aldrich syndrome protein Las17p interacts with the Arp2/3 complex
A Madania et al. Mol Biol Cell. 1999 Oct.
Free PMC article
Abstract
Yeast Las17 protein is homologous to the Wiskott-Aldrich Syndrome protein, which is implicated in severe immunodeficiency. Las17p/Bee1p has been shown to be important for actin patch assembly and actin polymerization. Here we show that Las17p interacts with the Arp2/3 complex. LAS17 is an allele-specific multicopy suppressor of ARP2 and ARP3 mutations; overexpression restores both actin patch organization and endocytosis defects in ARP2 temperature-sensitive (ts) cells. Six of seven ARP2 ts mutants and at least one ARP3 ts mutant are synthetically lethal with las17Delta ts confirming functional interaction with the Arp2/3 complex. Further characterization of las17Delta cells showed that receptor-mediated internalization of alpha factor by the Ste2 receptor is severely defective. The polarity of normal bipolar bud site selection is lost. Las17-gfp remains localized in cortical patches in vivo independently of polymerized actin and is required for the polarized localization of Arp2/3 as well as actin. Coimmunoprecipitation of Arp2p with Las17p indicates that Las17p interacts directly with the complex. Two hybrid results also suggest that Las17p interacts with actin, verprolin, Rvs167p and several other proteins including Src homology 3 (SH3) domain proteins, suggesting that Las17p may integrate signals from different regulatory cascades destined for the Arp2/3p complex and the actin cytoskeleton.
Figures
Figure 1
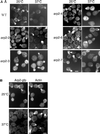
Actin phenotypes of ARP2 ts mutants. (A) Phalloidin staining of wild-type YMW201U(a) and mutant YMW221U, YMW231U, YMW241U, YMW261U, and YMW271U (a) strains containing ARP2 point mutations. Cells were grown to early log phase in complete medium at 25°C. Part of the culture was shifted to 37°C for 2 h. (B) Strain FDW23GK (a/α) containing an ARP2:GFP C-terminal fusion. Cells were treated as in A and then fixed and stained with TRITC-phalloidin as described in MATERIALS AND METHODS. The left panels were photographed with a GFP bandpass filter, and the right panels were photographed with a rhodamine filter. Bar, 5 μm.
Figure 2
LAS17 overexpression suppresses certain ARP2 ts mutants. Seven ARP2 ts strains (arp2-1 to 2-7/YMW211U–YMW271U) and wild-type (YMW201U) described in Table 2 were transformed with pGAD (vector) (A), pAMW171 (LAS17 in pGAD) (B), and pAMW200 (ARP2 in pGAD) (C). Transformants were streaked on SC − Ura and Leu plates and incubated at 37°C for 4 d. Note that the arp2-5 ts mutant grows at 37°C on minimal medium but is more ts on complete medium.
Figure 3
Suppression of arp2-2 actin and endocytosis phenotypes by LAS17. Strain YMW221U (bearing the arp2-2:URA3 ts allele) was transformed with vector (a), with pAMW171 (LAS17 2μ) (b), and with pAMW200 (ARP2 in pGAD) (c). (A) Transformants were grown at 25°C in liquid YPD to log phase and shifted to 37°C for 2 h. Cells were fixed, stained with TRITC-phalloidin, and photographed as described in MATERIALS AND METHODS. Bar, 5 μm. (B) Transformants were grown in liquid YPD at 25°C to log phase and incubated for 1 h with LY as described in MATERIALS AND METHODS. Cells were photographed using an FITC filter (LY) or Nomarski optics (differential interference contrast [DIC]).
Figure 4
LAS17 allele-specific suppression of ARP3 ts mutants. The arp3Δ (YMW301T) strain rescued by five different ARP3 ts alleles (arp3-9 to arp3-13 and ARP3) and strain YMW321L (bearing the arp3-14: LEU2 allele) were transformed by vector (a), pYEW170 (LAS17, 2μ) (b), or pCMW304 rescue plasmid (ARP3:LEU2) (c). Freshly cultured transformants were spotted onto YNB − Leu and Ura plates, which were incubated at 25°C for 5 d or at 36°C for 3 d before being photographed.
Figure 5
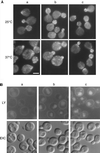
Endocytosis defects in las17Δ cells. (A) FM4-64 uptake by las17Δ cells. YMW171K (las17Δ) and YPH499 (WT) cells were grown to log phase in liquid YPD at 25°C and then concentrated and incubated for 15 min in 20 μM FM4-64 followed by a 45-min chase in fresh YPD. Cells were photographed using an FITC filter (top panel) or DIC optics (bottom panel). Bar, 5 μm. (B) Alpha factor internalization by las17Δ cells. las17Δ strain RH 4207 and its equivalent wild type were preincubated at 37°C for 15 min before the addition of 35S-alpha factor. The samples were processed as described by Dulic et al. (1991). Results are from one of two independent experiments, which gave nearly identical results. ●, WT, 24°C; ▪, las17Δ, 24°C; ○, WT, 37°C; □, las17Δ, 37°C.
Figure 6
Bipolar budding defects in las17Δ/las17Δ cells. Strain FMW173K was grown overnight in YPD at 25°C. Cells were fixed and stained with calcofluor, washed, and observed in the microscope with a UV filter. Bar, 5 μm.
Figure 7
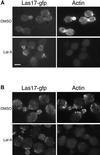
Functional Las17-gfp remains localized independently of actin. (A) Localization of Las17p at 25°C after treatment of cells with latrunculin-A. Strain YMW173G homozygous for LAS17:GFP was grown in liquid YPD to log phase at 25°C and incubated with latrunculin-A (100 μM final concentration) in DMSO or an equivalent volume of DMSO. Images show cells fixed after 30 min exposure to latrunculin-A, which were briefly fixed and then stained with Alexa-phalloidin. Bar, 5 μm. (B) Cells were grown as in A, fixed for 1 h, and then digested with zymolyase to make spheroplasts. Spheroplasts were labeled with rabbit anti-GFP IgG (left panels) and mouse monoclonal anti-actin IgG (right panels), which were revealed by goat anti-rabbit FITC and goat anti-mouse Cyanine 3 secondary antibodies using appropriate filters. No overlap between filter channels was detected in control exposures.
Figure 8
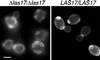
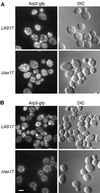
Loss of Arp2p and Arp3p polarity in las17Δ strains. (A) Localization of Arp2-gfp in wild-type and las17Δ/las17Δ cells. One allele of ARP2 was replaced by an ARP2:EGFP (Cormack et al., 1997) allele in FY1679 (WT) and in strain YMW173K (las17Δ/las17Δ) as described in MATERIALS AND METHODS. Cells were grown at 25°C in YPD, harvested, resuspended in 1 M sorbitol, and immediately put on slides for microscopic examination. Images were recorded using a GFP filter. (B) Localization of Arp3-gfp in wild-type and las17Δ/las17Δ cells. Experimental conditions were as in A. Bar, 5 μm.
Figure 9
Coimmunoprecipitation of Las17p and Arp2p (Arp2/3 complex). Las17-prot A was immunoprecipitated from crude extracts of strain YMW175K, which contained a chromosomally integrated allele of protein A-tagged Las17p and plasmid-borne HA tagged Arp2p (described in MATERIALS AND METHODS). After precipitation with TCA, cell extract and each step of the immunoprecipitation were subjected to 10% SDS-PAGE and blotted to nitrocellulose. E, extract; S, supernatant; L1, wash 1; L2, wash 2; L3, wash 3; P, immunoprecipitate; C, control immunoprecipitate, mock IP from crude extract of the YMW175K parental strain without protein A tag. (A) Las17-protA detected with purified rabbit IgG. (B) HA-tagged Arp2p detected with mouse monoclonal anti-HA IgG.
Similar articles
- Saccharomyces cerevisiae Bzz1p is implicated with type I myosins in actin patch polarization and is able to recruit actin-polymerizing machinery in vitro.
Soulard A, Lechler T, Spiridonov V, Shevchenko A, Shevchenko A, Li R, Winsor B. Soulard A, et al. Mol Cell Biol. 2002 Nov;22(22):7889-906. doi: 10.1128/MCB.22.22.7889-7906.2002. Mol Cell Biol. 2002. PMID: 12391157 Free PMC article. - The WASp homologue Las17p functions with the WIP homologue End5p/verprolin and is essential for endocytosis in yeast.
Naqvi SN, Zahn R, Mitchell DA, Stevenson BJ, Munn AL. Naqvi SN, et al. Curr Biol. 1998 Aug 27;8(17):959-62. doi: 10.1016/s0960-9822(98)70396-3. Curr Biol. 1998. PMID: 9742397 - Activation of the yeast Arp2/3 complex by Bee1p, a WASP-family protein.
Winter D, Lechler T, Li R. Winter D, et al. Curr Biol. 1999 May 6;9(9):501-4. doi: 10.1016/s0960-9822(99)80218-8. Curr Biol. 1999. PMID: 10322115 - WASP family proteins, more than Arp2/3 activators.
Tyler JJ, Allwood EG, Ayscough KR. Tyler JJ, et al. Biochem Soc Trans. 2016 Oct 15;44(5):1339-1345. doi: 10.1042/BST20160176. Biochem Soc Trans. 2016. PMID: 27911716 Free PMC article. Review. - Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins.
Higgs HN, Pollard TD. Higgs HN, et al. J Biol Chem. 1999 Nov 12;274(46):32531-4. doi: 10.1074/jbc.274.46.32531. J Biol Chem. 1999. PMID: 10551802 Review. No abstract available.
Cited by
- Aggregation and Prion-Inducing Properties of the G-Protein Gamma Subunit Ste18 are Regulated by Membrane Association.
Chernova TA, Yang Z, Karpova TS, Shanks JR, Shcherbik N, Wilkinson KD, Chernoff YO. Chernova TA, et al. Int J Mol Sci. 2020 Jul 16;21(14):5038. doi: 10.3390/ijms21145038. Int J Mol Sci. 2020. PMID: 32708832 Free PMC article. - An intact SH3 domain is required for myosin I-induced actin polymerization.
Geli MI, Lombardi R, Schmelzl B, Riezman H. Geli MI, et al. EMBO J. 2000 Aug 15;19(16):4281-91. doi: 10.1093/emboj/19.16.4281. EMBO J. 2000. PMID: 10944111 Free PMC article. - Regulation of the yeast amphiphysin homologue Rvs167p by phosphorylation.
Friesen H, Murphy K, Breitkreutz A, Tyers M, Andrews B. Friesen H, et al. Mol Biol Cell. 2003 Jul;14(7):3027-40. doi: 10.1091/mbc.e02-09-0613. Epub 2003 Apr 4. Mol Biol Cell. 2003. PMID: 12857883 Free PMC article. - Live-Cell Imaging of Mitochondria and the Actin Cytoskeleton in Budding Yeast.
Higuchi-Sanabria R, Swayne TC, Boldogh IR, Pon LA. Higuchi-Sanabria R, et al. Methods Mol Biol. 2016;1365:25-62. doi: 10.1007/978-1-4939-3124-8_2. Methods Mol Biol. 2016. PMID: 26498778 Free PMC article. - A novel single-cell screening platform reveals proteome plasticity during yeast stress responses.
Breker M, Gymrek M, Schuldiner M. Breker M, et al. J Cell Biol. 2013 Mar 18;200(6):839-50. doi: 10.1083/jcb.201301120. J Cell Biol. 2013. PMID: 23509072 Free PMC article.
References
- Amberg DC, Basart E, Botstein D. Defining protein interaction with yeast actin in vivo. Nat Struct Biol. 1995;2:28–35. - PubMed
- Amman AJ, Hong R. Disorders of the T-cell system. In: Stiehm ER, editor. Immunological Disorders in Children. Philadelphia: W.B. Saunders; 1989. pp. 257–315.
- Apenström P, Lindberg U, Hall A. Two GTPases, Cdc42 and Rac, bind directly to a protein implicated in the immunodeficiency disorder Wiskott–Aldrich syndrome. Curr Biol. 1996;6:70–75. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous