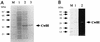Characterization of a new sigma-K-dependent peptidoglycan hydrolase gene that plays a role in Bacillus subtilis mother cell lysis - PubMed (original) (raw)
Characterization of a new sigma-K-dependent peptidoglycan hydrolase gene that plays a role in Bacillus subtilis mother cell lysis
F A Nugroho et al. J Bacteriol. 1999 Oct.
Abstract
Bacillus subtilis produces a 30-kDa peptidoglycan hydrolase, CwlH, during the late sporulation phase. Disruption of yqeE led to a complete loss of CwlH formation, indicating the identity of yqeE with cwlH. Northern blot analysis of cwlH revealed a 0.8-kb transcript after 6 to 7.5 h for the wild-type strain but not for the sigma(F), sigma(E), sigma(G), and sigma(K) mutants. Expression of the sigma(K)-dependent cwlH gene depended on gerE. Primer extension analysis also suggested that cwlH is transcribed by Esigma(K) RNA polymerase. CwlH produced in Escherichia coli harboring a cwlH plasmid is an N-acetylmuramoyl-L-alanine amidase (EC 3.5.1.28) and exhibited an optimum pH of 7.0 and high-level binding to the B. subtilis cell wall. A cwlC cwlH double mutation led to a lack of mother cell lysis even after 7 days of incubation in DSM medium, but the single mutations led to mother cell lysis after 24 h.
Figures
FIG. 1
Gene organization of yqeE (designated cwlH) and its flanking regions, and nucleotide sequence of the upstream region of cwlH. The peptides are numbered with respect to the translational start codon (+1) of each protein. −35 and −10 represent the −35 and −10 regions of a ςK promoter, and SD represents a Shine-Dalgarno sequence. The arrowhead indicates the transcriptional start point of cwlH. The GerE consensus sequence and restriction sites are shown by underlining and overlining, respectively, of the nucleotide sequence.
FIG. 2
SDS-polyacrylamide (12%) gel electrophoretic analysis of expression of the CwlH protein in E. coli(pREP4, pUCH2) (A) and zymography of CwlH with B. subtilis 168 cell wall (B). (A) E. coli M15(pREP4, pUCH2) cells were cultured in 100 ml of LB medium at 37°C. When the cell density reached 0.8 at OD600, 2 mM (final concentration) IPTG was added to the culture, followed by further incubation for 3 h. The cells were washed and sonicated, and then the broken-cell suspension was centrifuged. Proteins in the supernatant (equivalent to the 0.1 OD600 cells) were loaded onto lane 2. Lane 1 is a sample without IPTG induction. Lane 3 is the purified CwlH protein (7.5 μg) after nickel affinity column chromatography. M, marker proteins (Bio-Rad broad-range markers [1.5 μg of each]; from top to bottom, 97.4, 66.2, 45.0, 31.0, and 21.5 kDa). (B) The purified CwlH (1 μg) was loaded onto lanes 1 and 2. Lanes M (marker proteins) and 1, SDS-PAGE; lane 2, zymography with strain 168 cell wall. Zymographic patterns toward the M. luteus cell wall, 168 spore cortex, and muramic acid lactam-deficient EDD1 cortex were very similar to that toward the 168 cell wall (B, lane 2; reference 31).
FIG. 3
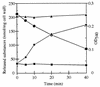
Digestion of B. subtilis cell wall with the purified CwlH protein. B. subtilis cell wall (2.0 mg) and the purified CwlH (9.6 μg) were mixed in 6 ml of a 1% potassium borate solution (pH 9.0) and then incubated at 37°C. Aliquots (500 μl of each) was removed at various intervals for determination of turbidity at 540 nm (●), DNP-diaminopimelic acid (▴), DNP-
l
-alanine (⧫), and DNP-
d
-alanine (■).
FIG. 4
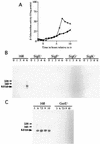
(A) β-Galactosidase activities of the cwlH-lacZ transcriptional fusion strains constructed in the B. subtilis chromosome. The strains were grown in DSM medium at 37°C. Squares, strain CWLHd; circles, strain 1G1Δ_cwlH_, diamonds, strain 168. (B and C) Northern hybridization analysis of cwlH with a specific RNA probe. B. subtilis total RNAs from cultured cells were purified with Isogen (numbers indicate the times [hours] after onset of sporulation), and 10 μg of each RNA was separated on a 1% formaldehyde agarose gel. Signals were detected with the digoxigenin-labeled RNA probe. The cwlH mRNA signal is indicated by a thick arrow; positions of 23S and 16S RNAs are indicated by thin arrows. 168, B. subtilis 168; SigE−, 1S60 (spoIIG); SigF−, 1S86 (spoIIA); SigG−, spoIIIGΔ1 (spoIIIG); SigK−, 1S38 (spoIIIC); GerE−, 1G12 (gerE).
FIG. 5
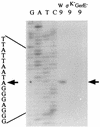
Determination of transcriptional start sites by primer extension analysis. Total RNAs (50 μg) at _t_9 from B. subtilis 168 (W), 1S38 (ςK−), and 1G12 (GerE−) were used as RNA samples. Signals were detected with 32P-labeled primer PEH1. Dideoxy DNA sequencing reaction mixtures with the same primer were electrophoresed in parallel (lanes G, A, T, and C). The arrows indicate the nucleotide at the transcriptional start site. The nucleotide sequence of the transcribed strand is given beside the sequence ladder.
FIG. 6
Zymographic analysis of cell extracts and culture supernatants of B. subtilis AC327 (wild type), ANC1 (cwlC), and ACH (cwlC cwlH) cells. Cultures at _t_10 were centrifuged, and then the supernatants (sup.) of the cultures (2 ml of each) and SDS extracts of the pellets were applied on a 12% polyacrylamide gel containing 0.1% B. subtilis cell wall. The cell wall lytic bands of CwlH and CwlC overlapped, as indicated by an arrow. Standard size markers are shown on the left.
FIG. 7
Phase-contrast micrographs of B. subtilis AC327 (wild type), ANB1 (cwlB), ANC1 (cwlC), CWLHd (cwlH), ABH (cwlB cwlH), ACH (cwlC cwlH), ABC (cwlB cwlC) and ABCH (cwlB cwlC cwlH) cells after 24 h incubation in DSM medium at 37°C. Bars, 10 μm.
FIG. 8
Alignment of amino acid sequences of class I _N_-acetylmuramoyl-
l
-alanine amidases. CwlA is a deduced phage lysin (17, 27), XlyA and XlyB are phage lysins from PBS1 (24, 5), and BlyA is a phage lysin from SPβ (36). Asterisks indicate identical amino acids among the five amidases in class I. Dashes indicate the introduction of gaps in the alignment, and numbers on the left are with respect to the N-terminal amino acid of each amidase.
Similar articles
- Novel Cell Wall Hydrolase CwlC from Bacillus thuringiensis Is Essential for Mother Cell Lysis.
Chen X, Gao T, Peng Q, Zhang J, Chai Y, Song F. Chen X, et al. Appl Environ Microbiol. 2018 Mar 19;84(7):e02640-17. doi: 10.1128/AEM.02640-17. Print 2018 Apr 1. Appl Environ Microbiol. 2018. PMID: 29374039 Free PMC article. - Molecular cloning of a sporulation-specific cell wall hydrolase gene of Bacillus subtilis.
Kuroda A, Asami Y, Sekiguchi J. Kuroda A, et al. J Bacteriol. 1993 Oct;175(19):6260-8. doi: 10.1128/jb.175.19.6260-6268.1993. J Bacteriol. 1993. PMID: 8407798 Free PMC article. - Characterization of the involvement of two compensatory autolysins in mother cell lysis during sporulation of Bacillus subtilis 168.
Smith TJ, Foster SJ. Smith TJ, et al. J Bacteriol. 1995 Jul;177(13):3855-62. doi: 10.1128/jb.177.13.3855-3862.1995. J Bacteriol. 1995. PMID: 7601853 Free PMC article. - Autolysins of Bacillus subtilis: multiple enzymes with multiple functions.
Smith TJ, Blackman SA, Foster SJ. Smith TJ, et al. Microbiology (Reading). 2000 Feb;146 ( Pt 2):249-262. doi: 10.1099/00221287-146-2-249. Microbiology (Reading). 2000. PMID: 10708363 Review. No abstract available. - Establishment of cell-specific transcription during sporulation in Bacillus subtilis.
Errington J, Illing N. Errington J, et al. Mol Microbiol. 1992 Mar;6(6):689-95. doi: 10.1111/j.1365-2958.1992.tb01517.x. Mol Microbiol. 1992. PMID: 1573998 Review.
Cited by
- Cytological analysis of the mother cell death process during sporulation in Bacillus subtilis.
Hosoya S, Lu Z, Ozaki Y, Takeuchi M, Sato T. Hosoya S, et al. J Bacteriol. 2007 Mar;189(6):2561-5. doi: 10.1128/JB.01738-06. Epub 2007 Jan 5. J Bacteriol. 2007. PMID: 17209033 Free PMC article. - Identification of SPOR domain amino acids important for septal localization, peptidoglycan binding, and a disulfide bond in the cell division protein FtsN.
Duncan TR, Yahashiri A, Arends SJ, Popham DL, Weiss DS. Duncan TR, et al. J Bacteriol. 2013 Dec;195(23):5308-15. doi: 10.1128/JB.00911-13. Epub 2013 Sep 20. J Bacteriol. 2013. PMID: 24056104 Free PMC article. - Programmed death in bacteria.
Lewis K. Lewis K. Microbiol Mol Biol Rev. 2000 Sep;64(3):503-14. doi: 10.1128/MMBR.64.3.503-514.2000. Microbiol Mol Biol Rev. 2000. PMID: 10974124 Free PMC article. Review. - Novel Cell Wall Hydrolase CwlC from Bacillus thuringiensis Is Essential for Mother Cell Lysis.
Chen X, Gao T, Peng Q, Zhang J, Chai Y, Song F. Chen X, et al. Appl Environ Microbiol. 2018 Mar 19;84(7):e02640-17. doi: 10.1128/AEM.02640-17. Print 2018 Apr 1. Appl Environ Microbiol. 2018. PMID: 29374039 Free PMC article. - Identification and characterization of a novel polysaccharide deacetylase C (PdaC) from Bacillus subtilis.
Kobayashi K, Sudiarta IP, Kodama T, Fukushima T, Ara K, Ozaki K, Sekiguchi J. Kobayashi K, et al. J Biol Chem. 2012 Mar 23;287(13):9765-9776. doi: 10.1074/jbc.M111.329490. Epub 2012 Jan 25. J Biol Chem. 2012. PMID: 22277649 Free PMC article.
References
- Blackman S, Smith T J, Foster S J. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology. 1998;144:73–82. - PubMed
- Canosi U, Morelli G, Trautner T A. The relationship between molecular structure and transformation efficiency of some Streptococcus aureus plasmids isolated from Bacillus subtilis. Mol Gen Genet. 1978;166:259–267. - PubMed
- da Silva E, Longchamp P F, Karamata D. Abstracts of the 9th International Conference on Bacilli. Lausanne, Switzerland: Universite de Lausanne; 1997. Identification of XlyB, the second lytic enzyme of the defective prophage PBSX, abstr. B25.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases