Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base - PubMed (original) (raw)
Construction of a pseudoreceptor that mediates transduction by adenoviruses expressing a ligand in fiber or penton base
D A Einfeld et al. J Virol. 1999 Nov.
Abstract
Modification of adenovirus to achieve tissue specific targeting for the delivery of therapeutic genes requires both the ablation of its native tropism and the introduction of specific, novel interactions. Inactivation of the native receptor interactions, however, would cripple the virus for growth in production cells. We have developed an alternative receptor, or pseudoreceptor, for the virus which might allow propagation of viruses with modified fiber proteins that no longer bind to the native adenovirus receptor (coxsackievirus/adenovirus receptor [CAR]). We have constructed a membrane-anchored single-chain antibody [m-scFv(HA)] which recognizes a linear peptide epitope (hemagglutinin [HA]). Incorporation of HA within the HI loop of the fiber protein enabled the modified virus to transduce pseudoreceptor expressing cells under conditions where fiber-CAR interaction was blocked or absent. The pseudoreceptor mediated virus transduction with an efficiency similar to that of CAR. In addition, the HA epitope mediated virus transduction through interaction with the m-scFv(HA) when it was introduced into penton base. These findings indicate that cells expressing the pseudoreceptor should support production of HA-tagged adenoviruses independent of retaining the fiber-CAR interaction. Moreover, they demonstrate that high-affinity targeting ligands may function following insertion into either penton base or fiber.
Figures
FIG. 1
Schematic diagram of scFv expression cassettes in pCAN-HA and pSc(HA). pCAN-HA carries the scFv for expression from the P_lac_ promoter. The scFv is flanked by an N-terminal signal sequence from gene 3 and a C-terminal E-tag epitope. The scFv was moved as an _Sfi_I/_Not_I fragment to pSc(HA) where expression is driven by the CMV promoter. The N-terminal signal sequence in this construct is from Igκ light chain, and the scFv is anchored in the membrane via the C-terminal PDGF receptor transmembrane (PDGFr TM) sequence from which it is separated by a Myc epitope spacer.
FIG. 2
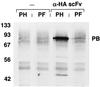
Western blot showing activity of soluble anti-HA scFv. Lysates from baculovirus-infected cells were incubated directly with anti-E antibody-coated agarose beads (−) or following preincubation with periplasmic extracts from E. coli transformed with pCAN-HA (α-HA scFv). These lysates were derived from cells infected with baculoviruses encoding HA-tagged (PH) or FLAG-tagged (PF) Ad penton base protein. Eluted proteins were subjected to SDS-PAGE, transferred to nitrocellulose, and probed with an antipenton antiserum. At the left are shown the positions of molecular weight standards in kilodaltons. The prominent band corresponding to epitope-tagged penton base is identified (PB).
FIG. 3
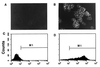
Binding of fluoresceinated HA peptide to m-scFv(HA)-expressing cells. 293-HA cells were observed by fluorescence microscopy following incubation with 100 nM HA* or the control peptide scrHA* (A and B). Cells were seeded to allow examination of discrete clusters of cells, and virtually all of the cells observed by light microscopy were found to fluoresce when exposed to HA*. For FACS analysis, cells detached in the presence of 5 mM EDTA were resuspended at 1.5 × 106 cells/ml and incubated with 100 nM HA* or scrHA* (C and D).
FIG. 4
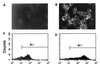
Specific inhibition of HA* binding to m-scFv(HA)-expressing cells by HA peptide. HA* peptide binding was performed as described for Fig. 3 except that cells were preincubated with unlabeled HA (A and C) or FLAG (B and D) peptide at 100 μM for 1 h and these remained present during incubation with HA*.
FIG. 5
Transduction of 293-HA cells by AdZ.F2K(HA) or AdZ.F2K(FLAG). Virus was added to cells at 1 FFU/cell and incubated for 1 h at 37°C before being replaced with fresh medium (Control). Prior to addition of virus, cells were incubated for 1 h with soluble Ad5 fiber at 5 μg/ml (F5), HA peptide at 100 μM (HA), a combination of the two (F5+HA), or a combination of 5 μg of Ad5 fiber per ml and 100 μM FLAG peptide (F5+FLAG). Infections were done as for the control but in the continued presence of competitor. At 20 h postinfection, cells were lysed and tested for β-galactosidase activity by using an ECL assay. After subtraction of background, the activities in the control samples for each virus were taken as 100% and those of the other samples were expressed relative to this. Shown are the means and error bars for the duplicate infections performed in this experiment.
FIG. 6
Transduction of CHO-HA cells by AdZ.F2K(HA). Infections of CHO-HA and CHO cells (at 5 FFU/cell) and β-galactosidase assays were performed as described for Fig. 5. To highlight the relative transduction of CHO-HA versus CHO cells, the results are shown as relative luminescence units (rlu), determined as the average from duplicate infections.
FIG. 7
Transduction of CHO cells with AdZ.F2K(HA) following exposure to AdCAR or AdSc(HA). CHO cells were incubated with AdCAR or AdSc(HA) at 104 particles (pu)/cell for 1 h. Twenty-four hours later, the cells were exposed to AdZ.F2K(HA) at 100 or 1,000 particles/cell for 1 h. Cell lysates were prepared 20 h later and assayed for β-galactosidase activity.
FIG. 8
Transduction of 293-HA cells by AdG.PB(HA) or AdG.PB(FLAG). Infections were done at 1 FFU/cell as described for Fig. 5. Cells were lysed at 20 h postinfection to assay for β-glucuronidase, and activities are expressed as percentages of those found for the unblocked control for each virus. Average values and error bars for duplicate infections are shown.
FIG. 9
Transduction of CHO-HA cells by AdG.PB(HA). CHO-HA and CHO cells were infected at 5 FFU/cell as described for Fig. 5. β-Glucuronidase activities are reported in relative light units (rlu) and shown as the average value for duplicate infections.
Similar articles
- Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and alphav integrin-binding ablation.
Koizumi N, Mizuguchi H, Sakurai F, Yamaguchi T, Watanabe Y, Hayakawa T. Koizumi N, et al. J Virol. 2003 Dec;77(24):13062-72. doi: 10.1128/jvi.77.24.13062-13072.2003. J Virol. 2003. PMID: 14645563 Free PMC article. - Simultaneous CAR- and alpha V integrin-binding ablation fails to reduce Ad5 liver tropism.
Martin K, Brie A, Saulnier P, Perricaudet M, Yeh P, Vigne E. Martin K, et al. Mol Ther. 2003 Sep;8(3):485-94. doi: 10.1016/s1525-0016(03)00182-5. Mol Ther. 2003. PMID: 12946322 - Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber.
Nakamura T, Sato K, Hamada H. Nakamura T, et al. J Virol. 2003 Feb;77(4):2512-21. doi: 10.1128/jvi.77.4.2512-2521.2003. J Virol. 2003. PMID: 12551989 Free PMC article. - Tropism and transduction of oncolytic adenovirus 5 vectors in cancer therapy: Focus on fiber chimerism and mosaicism, hexon and pIX.
Stepanenko AA, Chekhonin VP. Stepanenko AA, et al. Virus Res. 2018 Sep 15;257:40-51. doi: 10.1016/j.virusres.2018.08.012. Epub 2018 Aug 17. Virus Res. 2018. PMID: 30125593 Review. - Targeted adenovirus vectors.
Mizuguchi H, Hayakawa T. Mizuguchi H, et al. Hum Gene Ther. 2004 Nov;15(11):1034-44. doi: 10.1089/hum.2004.15.1034. Hum Gene Ther. 2004. PMID: 15610604 Review.
Cited by
- Protein transduction domains fused to virus receptors improve cellular virus uptake and enhance oncolysis by tumor-specific replicating vectors.
Kühnel F, Schulte B, Wirth T, Woller N, Schäfers S, Zender L, Manns M, Kubicka S. Kühnel F, et al. J Virol. 2004 Dec;78(24):13743-54. doi: 10.1128/JVI.78.24.13743-13754.2004. J Virol. 2004. PMID: 15564483 Free PMC article. - Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein.
Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V. Belousova N, et al. J Virol. 2002 Sep;76(17):8621-31. doi: 10.1128/jvi.76.17.8621-8631.2002. J Virol. 2002. PMID: 12163581 Free PMC article. - Biodistribution and retargeting of FX-binding ablated adenovirus serotype 5 vectors.
Alba R, Bradshaw AC, Coughlan L, Denby L, McDonald RA, Waddington SN, Buckley SM, Greig JA, Parker AL, Miller AM, Wang H, Lieber A, van Rooijen N, McVey JH, Nicklin SA, Baker AH. Alba R, et al. Blood. 2010 Oct 14;116(15):2656-64. doi: 10.1182/blood-2009-12-260026. Epub 2010 Jul 7. Blood. 2010. PMID: 20610817 Free PMC article. - Adenoviral vectors for improved gene delivery to the inner ear.
Praetorius M, Brough DE, Hsu C, Plinkert PK, Pfannenstiel SC, Staecker H. Praetorius M, et al. Hear Res. 2009 Feb;248(1-2):31-8. doi: 10.1016/j.heares.2008.11.009. Epub 2008 Dec 7. Hear Res. 2009. PMID: 19105978 Free PMC article. - Tropism-modification strategies for targeted gene delivery using adenoviral vectors.
Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, Baker AH. Coughlan L, et al. Viruses. 2010 Oct;2(10):2290-2355. doi: 10.3390/v2102290. Epub 2010 Oct 13. Viruses. 2010. PMID: 21994621 Free PMC article.
References
- Bergelson J M, Cunningham J A, Droguett G, Kurt-Jones E A, Krithivas A, Hong J S, Horwitz M S, Crowell R L, Finberg R W. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science. 1997;275:1320–1323. - PubMed
- Dmitriev I, Krasnykh V, Miller C R, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel D T. An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol. 1998;72:9706–9713. - PMC - PubMed
- Douglas J T, Rogers B E, Rosenfeld M E, Michael S I, Feng M, Curiel D T. Targeted gene delivery by tropism-modified adenoviral vectors. Nat Biotechnol. 1996;14:1574–1578. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources