Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2 - PubMed (original) (raw)
Comparative Study
. 1999 Oct 15;19(20):8778-88.
doi: 10.1523/JNEUROSCI.19-20-08778.1999.
K Schuette, R A Asher, A Dobbertin, S R Thornton, Y Calle-Patino, E Muir, J M Levine, H M Geller, J H Rogers, A Faissner, J W Fawcett
Affiliations
- PMID: 10516297
- PMCID: PMC6782756
- DOI: 10.1523/JNEUROSCI.19-20-08778.1999
Comparative Study
Comparing astrocytic cell lines that are inhibitory or permissive for axon growth: the major axon-inhibitory proteoglycan is NG2
P S Fidler et al. J Neurosci. 1999.
Abstract
Astrocytes, oligodendrocytes, and oligodendrocyte/type 2 astrocyte progenitors (O2A cells) can all produce molecules that inhibit axon regeneration. We have shown previously that inhibition of axon growth by astrocytes involves proteoglycans. To identify inhibitory mechanisms, we created astrocyte cell lines that are permissive or nonpermissive and showed that nonpermissive cells produce inhibitory chondroitin sulfate proteoglycans (CS-PGs). We have now tested these cell lines for the production and inhibitory function of known large CS-PGs. The most inhibitory line, Neu7, produces three CS-PGs in much greater amounts than the other cell lines: NG2, versican, and the CS-56 antigen. The contribution of NG2 to inhibition by the cells was tested using a function-blocking antibody. This allowed increased growth of dorsal root ganglion (DRG) axons over Neu7 cells and matrix and greatly increased the proportion of cortical axons able to cross from permissive A7 cells onto inhibitory Neu7 cells; CS-56 antibody had a similar effect. Inhibitory fractions of conditioned medium contained NG2 coupled to CS glycosaminoglycan chains, whereas noninhibitory fractions contained NG2 without CS chains. Enzyme preparations that facilitated axon growth in Neu7 cultures were shown to either degrade the NG2 core protein or remove CS chains. Versican is present as patches on Neu7 monolayers, but DRG axons do not avoid these patches. Therefore, NG2 appears to be the major axon-inhibitory factor made by Neu7 astrocytes. In the CNS, NG2 is expressed by O2A cells, which react rapidly after injury to produce a dense NG2-rich network, and by some reactive astrocytes. Our results suggest that NG2 may be a major obstacle to axon regeneration.
Figures
Fig. 1.
Sulfate incorporation into cell lysate (left 4 tracks) and supernatant (right 4 tracks) from Neu7 and A7 cells after growing the cells in the presence of Na235SO4overnight. Neu7 cells incorporate much more sulfate than A7 into both types of sample.
Fig. 2.
Fractionation of Neu7 conditioned medium on an anion exchange column (a). %B is salt concentration, mS is the conductivity, and_UV 280 nm_ is the UV absorption. The UV absorption curve shows that two absorption peaks come off the column, one at 0.5
m
NaCl and one at 1
m
NaCl. These two fractions and equivalent fractions from the A7 cell line were tested for their ability to inhibit axon growth on a laminin substrate. In_b_, the proportion of DRGs that grew axons on the four test substrates is shown. Only the 1
m
fraction from Neu7 was inhibitory.
Fig. 3.
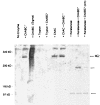
a, Production of NG2 by A7 and Neu7 cells. In A7 cells, NG2 is not detectable in the supernatant or membrane fraction. It is only detectable in small amounts in the highly concentrated 1
m
fraction from ion exchange fractionation. Neu7 produces large amounts of NG2 in cell surface and released forms, seen in the detergent lysate and culture supernatant samples. These samples were digested with chondroitinase ABC. B, NG2 in fractions from anion exchange. The fractions from both A7 and Neu7 coming off the column at 0.5
m
NaCl contain NG2, which forms a discrete band in the absence of chondroitinase ABC digestion, and therefore has little or no GAG chain attached. There is a large amount of NG2 in the 1
m
fraction from Neu7, but this cannot be seen as a discrete band unless it is digested with chondroitinase ABC, indicating that all the NG2 in this fraction carries GAG chains.
Fig. 4.
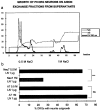
Axon growth from a postnatal DRG on an almost confluent culture of Neu7 cells. a is a neurofilament immunostain, and b is an NG2 immunostain. The axons had grown for 24 hr. Although most of the cells are stained for NG2, there is some unevenness in the intensity of NG2 staining on the Neu7 cells. However, during the 24 hr of the assay, the cells divide, migrate, and secrete NG2 into the medium, so correlations between axon path and NG2 staining are not meaningful.
Fig. 5.
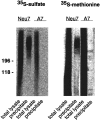
Immunoprecipitation of NG2 from biosynthetically labeled Neu7 and A7 cultures. Cell lines were labeled with35S-SO4 or 35S-methionine–cysteine as indicated. An aliquot of cell monolayer detergent extracts and the corresponding immunoprecipitates were resolved on neighboring lanes of a 4–10% SDS-PAGE slab gel. Note that polydisperse NG2 can be recovered from the Neu7 lysates of both types of labeling, whereas A7 does not yield a detectable signal on 35S-SO4and only a faint band without smear after35S-methionine–cysteine labeling. A phosphoimager picture of the gel is shown.
Fig. 6.
NG2 Western blot of Neu7 detergent lysate after digestion of samples with various enzymes. No NG2 band at ∼300 kDa is seen in the absence of digestion with chondroitinase ABC or AC, but after digestion with these enzymes, a sharp band is seen. Chondroitinase ABC and AC produce the same effect, and there is no additive effect. In Neu7 detergent lysate, a larger unidentified band at higher molecular weight is also seen. On digestion with keratanase, the 300 kDa band disappears, and a new band is seen at 100 kDa. When chondroitinase ABC is added to the keratanase, a 200 kDa band is seen in addition to the 100 kDa band. Digestion overnight with keratanase and chondroitinase (right lane) leads to almost complete disappearance of the 200 kDa band.
Fig. 7.
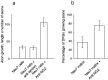
a, Axon growth from DRGs plated on Neu7 cells in the absence of antibodies, in the presence of 0.4 mg/ml of polyclonal anti-laminin, or 0.4 mg/ml of polyclonal anti-NG2. The growth measurement is the number of axons growing from each DRG multiplied by the length in millimeters. NG2 antibody considerably increases axon growth, whereas the anti-laminin antibody is without effect. b, DRGs plated on Neu7 extracellular matrix. The proportion of DRGs producing three or more axons was counted.
Fig. 8.
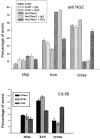
Anti-NG-2 significantly increases the ability of axons from cortical neurons to cross an A7–Neu7 cellular boundary. The behavior of 100 neurites per coverslip growing on A7 cells was assessed as they approached an Neu7 boundary, and classified as either stopped, turned, or crossed. In the presence of 0.2 mg/ml anti-NG2, the percentage of neurons that crossed onto Neu7 cells was increased from 10 to 48% (compare the two bars on the far right), whereas the number of neurites that turned was decreased from 75 to 39% (two bars on the_right_ of the turn measurements). There was no change in the percentage of neurites that crossed or turned after the addition of a control IgG. The CS-56 monoclonal antibody, which binds to particular motifs in CS chains, was also tested for its ability to affect axonal boundary crossing. In the presence of CS-56, the proportion of axons able to cross from Neu7 to A7 approximately doubled.
Fig. 9.
Production of versican by cell lines grown in FCS and horse serum (HS) and by primary astrocyte cultures purified by shaking off the top-dwelling cells. The Neu7 cell line produces two forms of versican, corresponding to the V0 and V1 variants, whereas the primary astrocyte cultures produce a smaller variant, corresponding to V2.
Fig. 10.
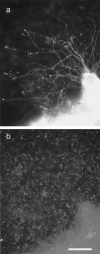
DRG axons growing over the surface of Neu7 cells, with the axons stained for neurofilament and the Neu7 cells stained for versican. The picture is a double exposure; axons and blobs are readily distinguishable. The versican is present in patches, with undetectable levels in between. The axons show no sign of avoiding the versican-rich areas.
Similar articles
- Increased axon regeneration in astrocytes grown in the presence of proteoglycan synthesis inhibitors.
Smith-Thomas LC, Stevens J, Fok-Seang J, Faissner A, Rogers JH, Fawcett JW. Smith-Thomas LC, et al. J Cell Sci. 1995 Mar;108 ( Pt 3):1307-15. doi: 10.1242/jcs.108.3.1307. J Cell Sci. 1995. PMID: 7622613 - Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition.
Jones LL, Sajed D, Tuszynski MH. Jones LL, et al. J Neurosci. 2003 Oct 15;23(28):9276-88. doi: 10.1523/JNEUROSCI.23-28-09276.2003. J Neurosci. 2003. PMID: 14561854 Free PMC article. - Limited growth of severed CNS axons after treatment of adult rat brain with hyaluronidase.
Moon LD, Asher RA, Fawcett JW. Moon LD, et al. J Neurosci Res. 2003 Jan 1;71(1):23-37. doi: 10.1002/jnr.10449. J Neurosci Res. 2003. PMID: 12478611 - Astrocytes and axon regeneration in the central nervous system.
Fawcett J. Fawcett J. J Neurol. 1994 Dec;242(1 Suppl 1):S25-8. doi: 10.1007/BF00939237. J Neurol. 1994. PMID: 7699404 Review. - The NG2 chondroitin sulfate proteoglycan: a multifunctional proteoglycan associated with immature cells.
Levine JM, Nishiyama A. Levine JM, et al. Perspect Dev Neurobiol. 1996;3(4):245-59. Perspect Dev Neurobiol. 1996. PMID: 9117258 Review.
Cited by
- Neurorepair and Regeneration of the Brain: A Decade of Bioscaffolds and Engineered Microtissue.
Zamproni LN, Mundim MTVV, Porcionatto MA. Zamproni LN, et al. Front Cell Dev Biol. 2021 Apr 7;9:649891. doi: 10.3389/fcell.2021.649891. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33898443 Free PMC article. Review. - Modulation of axonal regeneration in neurodegenerative disease: focus on Nogo.
Strittmatter SM. Strittmatter SM. J Mol Neurosci. 2002 Aug-Oct;19(1-2):117-21. doi: 10.1007/s12031-002-0021-7. J Mol Neurosci. 2002. PMID: 12212768 Review. - Inhibiting glycosaminoglycan chain polymerization decreases the inhibitory activity of astrocyte-derived chondroitin sulfate proteoglycans.
Laabs TL, Wang H, Katagiri Y, McCann T, Fawcett JW, Geller HM. Laabs TL, et al. J Neurosci. 2007 Dec 26;27(52):14494-501. doi: 10.1523/JNEUROSCI.2807-07.2007. J Neurosci. 2007. PMID: 18160657 Free PMC article. - Matrix metalloproteinase-9 facilitates remyelination in part by processing the inhibitory NG2 proteoglycan.
Larsen PH, Wells JE, Stallcup WB, Opdenakker G, Yong VW. Larsen PH, et al. J Neurosci. 2003 Dec 3;23(35):11127-35. doi: 10.1523/JNEUROSCI.23-35-11127.2003. J Neurosci. 2003. PMID: 14657171 Free PMC article. - Proteoglycans: road signs for neurite outgrowth.
Beller JA, Snow DM. Beller JA, et al. Neural Regen Res. 2014 Feb 15;9(4):343-55. doi: 10.4103/1673-5374.128235. Neural Regen Res. 2014. PMID: 25206822 Free PMC article.
References
- Asher R, Perides G, Vanderhaeghen J-J, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate–protein complex. J Neurosci Res. 1991;28:410–421. - PubMed
- Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. - PubMed
- Braunewell KH, Pesheva P, McCarthy JB, Furcht LT, Schmitz B, Schachner M. Functional involvement of sciatic nerve-derived versican and decorin-like molecules and other chondroitin sulfate proteoglycans in ecm-mediated cell-adhesion and neurite outgrowth. Eur J Neurosci. 1995;7:805–814. - PubMed
- Burg MA, Tillet E, Timpl R, Stallcup WB. Binding of the NG2 proteoglycan to type VI collagen and other extracellular matrix molecules. J Biol Chem. 1996;271:26110–26116. - PubMed
- Davies SJA, Fitch MT, Memberg SP, Hall AK, Raisman G, Silver J. Regeneration of adult axons in white matter tracts of the central nervous system. Nature. 1997;390:680–684. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous