Caveolin-1 modulates the activity of the volume-regulated chloride channel - PubMed (original) (raw)
Caveolin-1 modulates the activity of the volume-regulated chloride channel
D Trouet et al. J Physiol. 1999.
Abstract
1. Caveolae are small invaginations of the plasma membrane that have recently been implicated in signal transduction. In the present study, we have investigated whether caveolins, the principal protein of caveolae, also modulate volume-regulated anion channels (VRACs). 2. ICl,swell, the cell swelling-induced chloride current through VRACs, was studied in three caveolin-1-deficient cell lines: Caco-2, MCF-7 and T47D. 3. Electrophysiological measurements showed that ICl, swell was very small in these cells and that transient expression of caveolin-1 restored ICl,swell. The caveolin-1 effect was isoform specific: caveolin-1beta but not caveolin-1alpha upregulated VRACs. This correlated with a different subcellular distribution of caveolin-1alpha (perinuclear location) from caveolin-1beta (perinuclear and peripheral). 4. To explain the modulation of ICl, swell by caveolin-1 we propose that caveolin increases the availability of VRACs in the plasma membrane or, alternatively, that it plays a crucial role in the signal transduction cascade of VRACs.
Figures
Figure 1. Caveolin-1 is not expressed in Caco-2, MCF-7 and T47D cells
Fifty micrograms of Caco-2, MCF-7 and T47D cell lysates were separated on SDS-PAGE (12.5 % acrylamide), electroblotted and stained with a monoclonal antibody against caveolin-1. Human fibroblasts (left lane) and calf pulmonary artery endothelial cells (CPAE) were used as positive controls. The migration pattern of protein molecular mass standards (expressed in kDa) is shown on the right.
Figure 2. Transfection of caveolin-1 in Caco-2 cells restores _I_Cl,swell
Membrane currents before, during and after 25 % hypotonic stimulation (HTS) were recorded in a non-transfected Caco-2 cell (A-C) and in a Caco-2 cell transfected with caveolin-1 (D-F). A and D, time course of the current at +50 mV (upper trace) and at −100 mV (lower trace). During hypotonic stimulation (HTS), control cells developed only a small current with time (A), whereas in transfected cells a pronounced increase in membrane current is observed (D). The HTS-induced changes are reversible upon returning to isotonic conditions and completely disappear upon perfusion with a hypertonic solution (mann). B and E, I-V curves taken at the times marked by the filled symbols in A and D. The current-voltage relation of the membrane current at time zero in a typical control cell (a) and in a transfected cell (e) is compared to the respective I-V curves recorded during the plateau phase of the HTS-activated membrane current in the same control cell (b) and transfected cell (f). After switching back to isotonic conditions, the current returned to nearly control level (c and g). After application of a hypertonic mannitol solution (mann) the current reached its basal level (d and h). C and F, current traces during voltage steps applied at the time indicated by the asterisk in A and D. Note the different current scales. Voltage protocol: holding potential at −50 mV, steps between −100 and +100 mV, increment +20 mV.
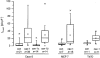
Figure 3. Effect of caveolin-1 isoforms on _I_Cl,swell in caveolin-1-deficient cell lines
Caco-2, MCF-7 and T47D cells, either control (con) or transfected with caveolin-1, −1α or −1β as indicated, were subjected to a 25 % HTS and _I_Cl,swell was measured. Difference currents (maximal HTS-triggered current minus basal current in isotonic medium) are plotted for the different conditions either at +50 mV (Caco-2) or at +80 mV (MCF-7 and T47D). The boxes correspond to interquartile ranges (25-75 percentile; middle line is median) with error bars representing the 5th (lower) and 95th percentile (upper). Small open square denotes the mean. Asterisks above and below boxes represent the 0th and 100th percentile. The differences between transfected and control conditions were statistically significant.
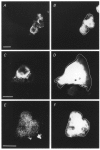
Figure 4. Immunofluorescence of Caco-2 cells transfected with caveolin-1 isoforms
Caco-2 cells were transfected with the bicistronic GFP expression vector containing caveolin-1 (A and B), −1α (C and D) or −1β (E and F). The GFP fluorescent signal (B, D and F) was used to identify transfected cells and to define the cell border (white superimposed line in GFP panels). Caveolin-1 isoforms were visualised using a non-discriminating monoclonal antibody (A, C and E) and phycoerythrin-conjugated secondary antibodies. Images representative for the different conditions indicate that caveolin-1, −1α and −1β are expressed. In addition, the subcellular distribution of caveolin-1α seems to be restricted to the perinuclear region, whereas the caveolin-1 and caveolin-1β signals reach the cell periphery. Scale bar corresponds to 10 μm in all panels.
Comment in
- A scaffolding for regulation of volume-sensitive Cl- channels.
Okada Y. Okada Y. J Physiol. 1999 Oct 1;520 Pt 1(Pt 1):2. doi: 10.1111/j.1469-7793.1999.00002.x. J Physiol. 1999. PMID: 10517793 Free PMC article. No abstract available.
Similar articles
- Inhibition of volume-regulated anion channels by expression of the cystic fibrosis transmembrane conductance regulator.
Vennekens R, Trouet D, Vankeerberghen A, Voets T, Cuppens H, Eggermont J, Cassiman JJ, Droogmans G, Nilius B. Vennekens R, et al. J Physiol. 1999 Feb 15;515 ( Pt 1)(Pt 1):75-85. doi: 10.1111/j.1469-7793.1999.075ad.x. J Physiol. 1999. PMID: 9925879 Free PMC article. - Expression of caveolin-1 and polarized formation of invaginated caveolae in Caco-2 and MDCK II cells.
Vogel U, Sandvig K, van Deurs B. Vogel U, et al. J Cell Sci. 1998 Mar;111 ( Pt 6):825-32. doi: 10.1242/jcs.111.6.825. J Cell Sci. 1998. PMID: 9472010 - De novo formation of caveolae in lymphocytes by expression of VIP21-caveolin.
Fra AM, Williamson E, Simons K, Parton RG. Fra AM, et al. Proc Natl Acad Sci U S A. 1995 Sep 12;92(19):8655-9. doi: 10.1073/pnas.92.19.8655. Proc Natl Acad Sci U S A. 1995. PMID: 7567992 Free PMC article. - Caveolae and caveolin isoforms in rat peritoneal macrophages.
Kiss AL, Turi A, Müller N, Kántor O, Botos E. Kiss AL, et al. Micron. 2002;33(1):75-93. doi: 10.1016/s0968-4328(00)00100-1. Micron. 2002. PMID: 11473817 Review. - Crowded little caves: structure and function of caveolae.
Schlegel A, Volonte D, Engelman JA, Galbiati F, Mehta P, Zhang XL, Scherer PE, Lisanti MP. Schlegel A, et al. Cell Signal. 1998 Jul;10(7):457-63. doi: 10.1016/s0898-6568(98)00007-2. Cell Signal. 1998. PMID: 9754713 Review.
Cited by
- LRRC8 Proteins Form Volume-Regulated Anion Channels that Sense Ionic Strength.
Syeda R, Qiu Z, Dubin AE, Murthy SE, Florendo MN, Mason DE, Mathur J, Cahalan SM, Peters EC, Montal M, Patapoutian A. Syeda R, et al. Cell. 2016 Jan 28;164(3):499-511. doi: 10.1016/j.cell.2015.12.031. Cell. 2016. PMID: 26824658 Free PMC article. - Cholesterol and ion channels.
Levitan I, Fang Y, Rosenhouse-Dantsker A, Romanenko V. Levitan I, et al. Subcell Biochem. 2010;51:509-49. doi: 10.1007/978-90-481-8622-8_19. Subcell Biochem. 2010. PMID: 20213557 Free PMC article. Review. - Ion channels and ion transporters of the transverse tubular system of skeletal muscle.
Jurkat-Rott K, Fauler M, Lehmann-Horn F. Jurkat-Rott K, et al. J Muscle Res Cell Motil. 2006;27(5-7):275-90. doi: 10.1007/s10974-006-9088-z. Epub 2006 Aug 24. J Muscle Res Cell Motil. 2006. PMID: 16933023 Review. - Caveolin-1 and -2 interact with connexin43 and regulate gap junctional intercellular communication in keratinocytes.
Langlois S, Cowan KN, Shao Q, Cowan BJ, Laird DW. Langlois S, et al. Mol Biol Cell. 2008 Mar;19(3):912-28. doi: 10.1091/mbc.e07-06-0596. Epub 2007 Dec 27. Mol Biol Cell. 2008. PMID: 18162583 Free PMC article. - Regulation of the cellular content of the organic osmolyte taurine in mammalian cells.
Lambert IH. Lambert IH. Neurochem Res. 2004 Jan;29(1):27-63. doi: 10.1023/b:nere.0000010433.08577.96. Neurochem Res. 2004. PMID: 14992263 Review.
References
- Altenberg GA, Deitmer JW, Glass DC, Reuss L. P-glycoprotein-associated Cl− currents are activated by cell swelling but do not contribute to cell volume regulation. Cancer Research. 1994;54:618–622. - PubMed
- Anderson RGW. The caveolae membrane system. Annual Review of Biochemistry. 1998;67:199–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources