Visualizing the kinetics of tumor-cell clearance in living animals - PubMed (original) (raw)
Visualizing the kinetics of tumor-cell clearance in living animals
T J Sweeney et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Evaluation of potential antineoplastic therapies would be enhanced by noninvasive detection of tumor cells in living animals. Because light is transmitted through mammalian tissues, it was possible to use bioluminescence to monitor (both externally and quantitatively) growth and regression of labeled human cervical carcinoma (HeLa) cells engrafted into immunodeficient mice. The efficacy of both chemotherapy and immunotherapeutic treatment with ex vivo expanded human T cell-derived effector cells was evaluated. In the absence of therapy, animals showed progressive increases in signal intensity over time. Animals treated with cisplatin had marked reductions in tumor signal; 5'-fluorouracil was less effective, and cyclophosphamide was ineffective. Immunotherapy dramatically reduced signals at high effector-to-target cell ratios, and significant decreases were observed with lower ratios. This model system allowed sensitive, quantitative, real-time spatiotemporal analyses of the dynamics of neoplastic cell growth and facilitated rapid optimization of effective treatment regimens.
Figures
Figure 1
Sensitivity of detection of HeLa-luc cells in culture and in animals. (A and B) Dilution series of HeLa-luc cells were imaged in 96-well cell culture plates over a range of 0–1,000 cells. Photon counts from each of the triplicate wells were determined with a 5-min integration time, and the means of total photon counts were plotted with respect to cell number. (C) CB-17 SCID mice were irradiated with 200 cGy to eradicate residual natural killer activity. HeLa-luc cells, in a series ranging from 25 cells to 2.5 × 108 cells, were injected into the mice (i.p.). Groups of 3–6 mice at each cell concentration (except for 2.5 × 108 cells, which represents a single animal) were used to establish the limits of detection in vivo. Only the animals injected with the fewest cells are displayed here to show the sensitivity of the detection method. (D) Signals from the entire abdominal region of each mouse were quantified, and the mean photon counts were plotted. There was limited variation for each of the data points, which was <0.1% for all data points, with the exception of 2.5 × 105 cells, which was <5%.
Figure 2
Tumor-cell growth in animals with and without therapy. SCID mice, 6–8 weeks old, were irradiated with 200 cGy to eradicate functional natural killer cell activity (day 0). The following day, the mice received i.p. injections of 1 × 104 HeLa-luc cells, and baseline images and intensities were obtained. The mice were then treated with cyclophosphamide, 5′-FU, or PBS on day 1 as control. Tumor progression was monitored by measuring light emission from individual mice every 7 days for a period of 28 days. Growth of tumors in individual treated mice is shown with black lines, and the range of tumor growth in the absence of therapy is shown by the gray area. Growth curves designated by letters (A–C) indicate those tumor-growth profiles that show a range of therapeutic responses that were selected for presentation in Figs. 3 and 4. In the immunotherapy treatment groups, SCID mice received either CIK cells or PBS as control.
Figure 3
Tumor dynamics after chemotherapy in vivo. Images representing the entire time course for selected animals in each treatment group are shown; letters correspond to plotted data designated A–C in Fig. 2. Three representative animals were selected from the PBS control group. Profiles of animals with tumor-growth trajectories representing the full range of therapeutic response are shown for the 5-FU and cisplatin groups. Images representing the full time course for each animal are displayed identically at a bit range of 0–3 (first five columns) to show the exponential increases indicated in Fig. 2. The last image, obtained at day 28, is displayed again at a bit range of 0–7 to reveal the anatomic localization of the signals (last column).
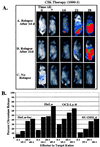
Figure 4
CIK immunotherapy in vivo and response of cell lines to CIK cells in vitro. (A) Time courses for animals were selected to show the full spectrum of response to immunotherapy. Animals A and B show relapse of tumor growth after days 14 and 21, respectively. Animal C shows complete eradication of tumor signal throughout the 28-day experimental period. (B) In vitro cytotoxicity of CIK cells against HeLa was not altered by luciferase expression. Other cell lines are shown as representative targets for CIK cells.
Figure 5
PCR analyses of long-term survivors in the cisplatin-treated group. Mice were evaluated by PCR to determine whether DNA from the xenograft could be detected in the absence of a detectable bioluminescent signal. PCR with two sets of nested primers for the luciferase gene was performed on DNA from cells obtained from the peritoneal cavity.
References
- Cavé H, van der Werff ten Bosch J, Suciu S, Guidal C, Waterkeyn C, Otten J, Bakkus M, Thielemans K, Grandchamp B, Vilmer E. N Engl J Med. 1998;339:591–598. - PubMed
- Hirsch-Ginsberg C. Annu Rev Med. 1998;49:111–122. - PubMed
- Kamel-Reid S, Latarte M, Sirard C, Doedens M, Grunberger T, Fulop G, Freedman M H, Phillips R A, Dick J E. Science. 1989;246:1597–1600. - PubMed
- Takahashi H, Nakada T, Puisieux I. Science. 1993;259:1460–1464. - PubMed
- Uckun F M. Blood. 1996;88:1135–1146. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources