Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines - PubMed (original) (raw)
Immunoglobulin A1 protease, an exoenzyme of pathogenic Neisseriae, is a potent inducer of proinflammatory cytokines
D R Lorenzen et al. J Exp Med. 1999.
Abstract
A characteristic of human pathogenic Neisseriae is the production and secretion of an immunoglobulin (Ig)A1-specific serine protease (IgA1 protease) that cleaves preferentially human IgA1 and other target proteins. Here we show a novel function for native IgA1 protease, i.e., the induction of proinflammatory cytokines such as tumor necrosis factor (TNF)-alpha, interleukin (IL)-1beta, IL-6, and IL-8 from peripheral blood mononuclear cells. The capacity of IgA1 protease to elicit such cytokine responses in monocytes was enhanced in the presence of T lymphocytes. IgA1 protease did not induce the regulatory cytokine IL-10, which was, however, found in response to lipopolysaccharide and phytohemagglutinin. The immunomodulatory effects caused by IgA1 protease require a native form of the enzyme, and denaturation abolished cytokine induction. However, the proteolytic activity is not required for the cytokine induction by IgA1 protease. Our results indicate that IgA1 protease exhibits important immunostimulatory properties and may contribute substantially to the pathogenesis of neisserial infections by inducing large amounts of TNF-alpha and other proinflammatory cytokines. In particular, IgA1 protease may represent a key virulence determinant of bacterial meningitis.
Figures
Figure 1
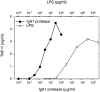
Purified IgA1 protease induces dose-dependent release of TNF-α from PBMCs. On the top axis, LPS concentrations were set according to the endotoxin content in the IgA1 protease protein on the bottom axis. TNF-α synthesis induced by LPS and IgA1 protease is shown by ▿ and •, respectively. PBMCs were cultured for 18 h in the presence of IgA1 protease or LPS. TNF-α was measured in pooled cell-free culture supernatants by a specific ELISA. TNF-α levels in nonstimulated control cells (medium control) did not exceed 0.02 ng/ml. Data were confirmed by three experiments using PBMCs from different donors.
Figure 2
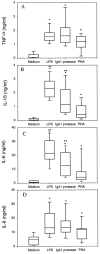
Comparison of cytokine production by PBMCs from different donors. PBMCs were cultured under low endotoxin conditions (<10 pg/ml as determined by the Limulus amebocyte lysate [LAL] assay). The indicated stimuli were added to the medium at the following concentrations: LPS, 30 ng/ml; PHA, 5 μg/ml; and IgA1 protease, 10 μg/ml. The cell-free supernatants were harvested after 18 h, pooled, and analyzed for the indicated cytokines by ELISA. Plots include data from n = 15 (for TNF-α [A] and IL-6 [C]) or n = 12 (for IL-1β [B] and IL-8 [D]) independent experiments. Boxes represent second and third quartiles; the lines within each box are the median values; the error bars represent 5 and 95% confidence. To compare cytokine levels in the different stimulation groups, the nonparametric Wilcoxon rank test was used. Results are considered as significantly different from values obtained with medium alone (*P < 0.05) and as significantly different from PHA (**P < 0.01).
Figure 3
Production of proinflammatory cytokines by purified monocytes in medium alone (white bars) and in response to 30 ng/ml LPS (hatched bars) or 10 μg/ml IgA1 protease (black bars). The level of each cytokine in the supernatant of stimulated monocyte cultures (105) was determined after 18 h. The results shown here are mean values from three different experiments ± SEM.
Figure 4
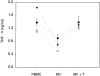
PBLs enhance TNF-α release of monocytes. PBMCs were separated by counterflow elutriation and adherence into monocytes (Mn) and lymphocytes (Ly) as described in Materials and Methods. After 1 h incubation, the autologous lymphocytes were added to the monocytes at a monocyte/lymphocyte ratio of 1:2. Fixed lymphocytes were treated with 2% paraformaldehyde (15 min at 0°C). TNF-α levels in the supernatants were measured 18 h after stimulation with 30 ng/ml LPS, 5 μg/ml PHA, or 10 μg/ml IgA1 protease.
Figure 7
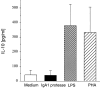
IL-10 release by PBMCs stimulated with IgA1 protease (10 μg/ml), LPS (30 ng/ml), or PHA (5 μg/ml) after 18 h incubation. Culture medium alone was used as a control. The means ± SEM of five independent experiments with PBMCs from five different donors are shown.
Figure 5
Purified CD3+ T lymphocytes enhance TNF-α production of CD14+ monocytes. The autologous CD3+ T lymphocytes (T) were added to the monocytes (Mn) at a monocyte/lymphocyte ratio of 1:4. TNF-α levels in the supernatants were measured after 18 h of culture with 10 μg/ml IgA1 protease. The results shown here are mean values from three experiments from different donors ± SEM (▾, ○, and •).
Figure 6
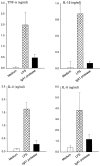
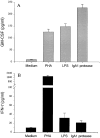
GM-CSF (A) and IFN-γ (B) release of PBMCs in response to IgA1 protease (10 μg/ml), PHA (5 μg/ml), and LPS (30 ng/ml). The levels of these cytokines were determined in supernatants after 18 h. The results (means ± SD of three determinations) were confirmed by three independent experiments.
Similar articles
- Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans.
Tsirpouchtsidis A, Hurwitz R, Brinkmann V, Meyer TF, Haas G. Tsirpouchtsidis A, et al. Infect Immun. 2002 Jan;70(1):335-44. doi: 10.1128/IAI.70.1.335-344.2002. Infect Immun. 2002. PMID: 11748199 Free PMC article. - IgA1 protease from Neisseria gonorrhoeae inhibits TNFalpha-mediated apoptosis of human monocytic cells.
Beck SC, Meyer TF. Beck SC, et al. FEBS Lett. 2000 Apr 28;472(2-3):287-92. doi: 10.1016/s0014-5793(00)01478-2. FEBS Lett. 2000. PMID: 10788628 - [Biological significance of IgA1 proteases].
Zhang Z, Li Q, Fan J. Zhang Z, et al. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011 Apr;28(2):423-8. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2011. PMID: 21604515 Review. Chinese. - Uptake and nuclear transport of Neisseria IgA1 protease-associated alpha-proteins in human cells.
Pohlner J, Langenberg U, Wölk U, Beck SC, Meyer TF. Pohlner J, et al. Mol Microbiol. 1995 Sep;17(6):1073-83. doi: 10.1111/j.1365-2958.1995.mmi_17061073.x. Mol Microbiol. 1995. PMID: 8594327 - Proteases of the pathogenic neisseriae: possible role in infection.
O'Reilly TM, Bhatti AR. O'Reilly TM, et al. Microbios. 1986;45(183):113-29. Microbios. 1986. PMID: 3086672 Review.
Cited by
- Characterization of igaB, a second immunoglobulin A1 protease gene in nontypeable Haemophilus influenzae.
Fernaays MM, Lesse AJ, Cai X, Murphy TF. Fernaays MM, et al. Infect Immun. 2006 Oct;74(10):5860-70. doi: 10.1128/IAI.00796-06. Infect Immun. 2006. PMID: 16988265 Free PMC article. - Neisserial immunoglobulin A1 protease induces specific T-cell responses in humans.
Tsirpouchtsidis A, Hurwitz R, Brinkmann V, Meyer TF, Haas G. Tsirpouchtsidis A, et al. Infect Immun. 2002 Jan;70(1):335-44. doi: 10.1128/IAI.70.1.335-344.2002. Infect Immun. 2002. PMID: 11748199 Free PMC article. - Virulence functions of autotransporter proteins.
Henderson IR, Nataro JP. Henderson IR, et al. Infect Immun. 2001 Mar;69(3):1231-43. doi: 10.1128/IAI.69.3.1231-1243.2001. Infect Immun. 2001. PMID: 11179284 Free PMC article. Review. No abstract available. - Transcriptome analysis of Neisseria meningitidis during infection.
Dietrich G, Kurz S, Hübner C, Aepinus C, Theiss S, Guckenberger M, Panzner U, Weber J, Frosch M. Dietrich G, et al. J Bacteriol. 2003 Jan;185(1):155-64. doi: 10.1128/JB.185.1.155-164.2003. J Bacteriol. 2003. PMID: 12486052 Free PMC article.
References
- Zenilman J.M. Gonorrheaclinical and public health issues. Hosp. Pract. 1993;28:29–35. - PubMed
- Cartwright K.A., Ala'Aldeen D.A. Neisseria meningitidisclinical aspects. J. Infect. 1997;34:15–19. - PubMed
- Cannon J.G., Sparling P.F. The genetics of the gonococcus. Annu. Rev. Microbiol. 1984;38:111–133. - PubMed
- Tramont E.C., Boslego J.W., Chung R. Parenteral gonococcal virus vaccine. In: Schoolnick G.K., Brooks G.F., Falkow S., editors. The Pathogenic Neisseria. American Society for Microbiology; Washington, DC: 1985. pp. 316–327.
- Meyer T.F., Pohlner J., van Putten J.P. Biology of the pathogenic Neisseriae . Curr. Top. Microbiol. Immunol. 1994;192:283–317. - PubMed