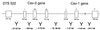Caveolins, liquid-ordered domains, and signal transduction - PubMed (original) (raw)
Review
Caveolins, liquid-ordered domains, and signal transduction
E J Smart et al. Mol Cell Biol. 1999 Nov.
No abstract available
Figures
FIG. 1
Caveolae and caveola-related domains. A diagram summarizing the various biochemical, morphological, and functional features of these plasma membrane-associated microdomains is shown. See text for details. DIGs, detergent-insoluble glycolipid-rich membranes; GEMs, glycolipid-enriched membranes; GPCRs, G-protein-coupled receptors; RTKs, receptor tyrosine kinases; NRTKs, nonreceptor tyrosine kinases; PLDs, phospholipase D.
FIG. 2
Dynamin localizes to caveolae in cultured epithelial cells. (A) Fluorescence micrographs representing laser scanning confocal microscopy of cultured hepatocytes that were double-labeled with a monoclonal anti-caveolin-1 antibody as a marker for caveolae (a) and a polyclonal antibody to dynamin to label the endogenous dynamin (b). A significant number of vesicular structures are labeled with both antibodies (arrows and outlined areas), indicating colocalization of dynamin and caveolin-1. Bar, 8.0 μm. (B) Horseradish peroxidase-cholera toxin B is sequestered within caveolae in dynamin-inhibited cells. Electron micrographs showing hepatocytes that were injected with an inhibitory dynamin antibody and incubated with peroxidase-conjugated cholera toxin are shown. Most of the toxin, represented by the peroxidase reaction product, has not been internalized but instead resides on the plasma membrane grape-like caveolar clusters. Bars, 0.2 μm.
FIG. 2
Dynamin localizes to caveolae in cultured epithelial cells. (A) Fluorescence micrographs representing laser scanning confocal microscopy of cultured hepatocytes that were double-labeled with a monoclonal anti-caveolin-1 antibody as a marker for caveolae (a) and a polyclonal antibody to dynamin to label the endogenous dynamin (b). A significant number of vesicular structures are labeled with both antibodies (arrows and outlined areas), indicating colocalization of dynamin and caveolin-1. Bar, 8.0 μm. (B) Horseradish peroxidase-cholera toxin B is sequestered within caveolae in dynamin-inhibited cells. Electron micrographs showing hepatocytes that were injected with an inhibitory dynamin antibody and incubated with peroxidase-conjugated cholera toxin are shown. Most of the toxin, represented by the peroxidase reaction product, has not been internalized but instead resides on the plasma membrane grape-like caveolar clusters. Bars, 0.2 μm.
FIG. 3
The caveolin gene family. An alignment of the protein sequences of murine caveolin-1, -2, and -3 is shown. Identical residues are boxed and highlighted. Note that caveolin-1 and -3 are most closely related, while caveolin-2 is divergent. Translation initiation sites are circled. In addition, the positions of the membrane-spanning segment (green) and the oligomerization domain (purple) are indicated.
FIG. 4
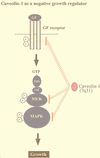
Caveolins negatively regulate signaling along the p42/44 MAPK cascade. Caveolae have been implicated in signaling through the p42/44 MAPK pathway. Caveolin-1 can inhibit signal transduction from the p42/44 MAPK cascade both in vitro and in vivo by acting as a natural endogenous inhibitor of EGF-R, MEK, and ERK (31). Conversely, when NIH 3T3 cells are used, antisense-mediated reductions in caveolin-1 protein expression are sufficient to constitutively activate the p42/44 MAPK cascade and drive oncogenic transformation (49). In normal NIH 3T3 cells, caveolin-1 expression levels are downregulated in rapidly dividing cells and dramatically upregulated at confluency. Thus, upregulation of caveolin-1 expression levels may be important in mediating normal contact inhibition and in negatively regulating the activation state of the p42/44 MAPK cascade. In accordance with these findings, the caveolin-1 gene is localized to a suspected tumor suppressor locus that is deleted in many forms of human cancer (7q31.1/D7S 522 locus) (34, 36).
FIG. 5
Detailed organization of the human caveolin-1 and -2 locus and its relationship to D7S 522, a microsatellite marker that is deleted in many forms of human cancer. The sizes of the exons and the distances between them are indicated. Note that the marker D7S 522 is located ∼67 kb upstream of the caveolin-2 gene and that the caveolin-2 gene is located ∼19 kb upstream of the caveolin-1 gene.
FIG. 6
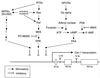
Schematic diagram summarizing the signaling pathways that down-regulate caveolin-1 gene expression via transcriptional control. The points of control that are affected by oncogenic activating mutations (Ras, Raf, Src, and Abl) and pharmacological agents (PD 98059, forskolin, and IBMX) are as indicated. RTKs, receptor tyrosine kinases; cAMP, cyclic AMP; NRTKs, nonreceptor tyrosine kinases; GPCRs, G-protein-coupled receptors; PDE, cyclic nucleotide phosphodiesterase. The overall structure of the murine caveolin-1 gene is as we described previously (–36).
FIG. 7
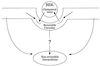
Caveolae mediate SR-BI-dependent uptake of cholesterol esters from HDL. HDL cholesterol esters are initially associated with plasma membrane caveolae. While in caveolae, cholesterol esters may either efflux back to HDL or translocate to nonreversible pools within the plasma membrane or an intracellular membrane compartment. The mechanism for internalization of caveolar cholesterol esters remains unknown.
Similar articles
- Caveolin, cholesterol and Ras signalling.
Sternberg PW, Schmid SL. Sternberg PW, et al. Nat Cell Biol. 1999 Jun;1(2):E35-7. doi: 10.1038/10028. Nat Cell Biol. 1999. PMID: 10559891 No abstract available. - [Physiological importance of plasmalemmal caveola].
Fujimoto T. Fujimoto T. Seikagaku. 1995 Dec;67(12):1396-401. Seikagaku. 1995. PMID: 8618076 Review. Japanese. No abstract available. - [Caveolae membrane domains, specialized transmembrane exchange zones implicated in cell signalling].
Roch-Arveiller M, Couderc R. Roch-Arveiller M, et al. Ann Biol Clin (Paris). 2000 Mar-Apr;58(2):141-6. Ann Biol Clin (Paris). 2000. PMID: 10760700 Review. French. - Caveolae and caveolins.
Parton RG. Parton RG. Curr Opin Cell Biol. 1996 Aug;8(4):542-8. doi: 10.1016/s0955-0674(96)80033-0. Curr Opin Cell Biol. 1996. PMID: 8791446 Review. - Crowded little caves: structure and function of caveolae.
Schlegel A, Volonte D, Engelman JA, Galbiati F, Mehta P, Zhang XL, Scherer PE, Lisanti MP. Schlegel A, et al. Cell Signal. 1998 Jul;10(7):457-63. doi: 10.1016/s0898-6568(98)00007-2. Cell Signal. 1998. PMID: 9754713 Review.
Cited by
- Caveolin-1 Regulates the P2Y2 Receptor Signaling in Human 1321N1 Astrocytoma Cells.
Martinez NA, Ayala AM, Martinez M, Martinez-Rivera FJ, Miranda JD, Silva WI. Martinez NA, et al. J Biol Chem. 2016 Jun 3;291(23):12208-22. doi: 10.1074/jbc.M116.730226. Epub 2016 Apr 18. J Biol Chem. 2016. PMID: 27129210 Free PMC article. - Caveolin-1 deficiency impairs synaptic transmission in hippocampal neurons.
Koh S, Lee W, Park SM, Kim SH. Koh S, et al. Mol Brain. 2021 Mar 16;14(1):53. doi: 10.1186/s13041-021-00764-z. Mol Brain. 2021. PMID: 33726791 Free PMC article. - White matter rafting--membrane microdomains in myelin.
Debruin LS, Harauz G. Debruin LS, et al. Neurochem Res. 2007 Feb;32(2):213-28. doi: 10.1007/s11064-006-9137-4. Epub 2006 Sep 21. Neurochem Res. 2007. PMID: 17031566 Review. - Hypoxic stress disrupts HGF/Met signaling in human trophoblasts: implications for the pathogenesis of preeclampsia.
Li G, Wang Y, Cao G, Ma Y, Li YX, Zhao Y, Shao X, Wang YL. Li G, et al. J Biomed Sci. 2022 Feb 3;29(1):8. doi: 10.1186/s12929-022-00791-5. J Biomed Sci. 2022. PMID: 35114998 Free PMC article. - High-resolution proton NMR measures mobile lipids associated with Triton-resistant membrane domains in haematopoietic K562 cells lacking or expressing caveolin-1.
Ferretti A, Knijn A, Raggi C, Sargiacomo M. Ferretti A, et al. Eur Biophys J. 2003 May;32(2):83-95. doi: 10.1007/s00249-002-0273-8. Epub 2003 Jan 28. Eur Biophys J. 2003. PMID: 12734696
References
- Anderson R G W. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. - PubMed
- Anderson R G W, Kamen B A, Rothberg K G, Lacey S W. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. - PubMed
- Babitt J, Trigatti B, Rigotti A, Smart E J, Anderson R G, Xu S, Krieger M. Murine SR-BI, a high density lipoprotein receptor that mediates selective lipid uptake, is N-glycosylated and fatty acylated and colocalizes with plasma membrane caveolae. J Biol Chem. 1997;272:13242–13249. - PubMed
- Bickel P E, Scherer P E, Schnitzer J E, Oh P, Lisanti M P, Lodish H F. Flotillin and epidermal surface antigen define a new family of caveolae-associated integral membrane proteins. J Biol Chem. 1997;272:13793–13802. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL-58475/HL/NHLBI NIH HHS/United States
- HL-62844/HL/NHLBI NIH HHS/United States
- CA-80250/CA/NCI NIH HHS/United States
- T32 GM007288/GM/NIGMS NIH HHS/United States
- R01 CA080250/CA/NCI NIH HHS/United States
- R01 HL058475/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources