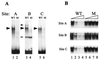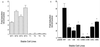Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D-responsive transcription and contribute to chromatin organization - PubMed (original) (raw)
Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D-responsive transcription and contribute to chromatin organization
A Javed et al. Mol Cell Biol. 1999 Nov.
Abstract
Three Cbfa motifs are strategically positioned in the bone-specific rat osteocalcin (rOC) promoter. Sites A and B flank the vitamin D response element in the distal promoter and sites B and C flank a positioned nucleosome in the proximal promoter. The functional significance of each Cbfa element was addressed by mutating individual or multiple Cbfa sites within the context of the -1.1-kb rOC promoter fused to a chloramphenicol acetyltransferase reporter gene. Promoter activity was assayed following transient transfection and after stable genomic integration in ROS 17/2.8 osteoblastic cell lines. We show that all three Cbfa sites are required for maximal basal expression of the rOC promoter. However, the distal sites A and B each contribute significantly more (P < 0.001) to promoter activity than site C. In a genomic context, sites A and B can largely compensate for a mutation at the proximal site C, and paired mutations involving site A (mAB or mAC) result in a far greater loss of activity than the mBC mutation. Strikingly, mutation of the three Cbfa sites leads to abrogation of responsiveness to vitamin D. Vitamin D-enhanced activity is also not observed when sites A and B are mutated. Significantly, related to these losses in transcriptional activity, mutation of the three Cbfa sites results in altered chromatin structure as reflected by loss of DNase I-hypersensitive sites at the vitamin D response element and over the proximal tissue-specific basal promoter. These findings strongly support a multifunctional role for Cbfa factors in regulating gene expression, not only as simple transcriptional transactivators but also by facilitating modifications in promoter architecture and chromatin organization.
Figures
FIG. 1
Wild-type and mutated Cbfa motifs in the rat OC gene promoter. The positions and nucleotide numbers of the three Cbfa sites (A, B, and C) relative to the VDRE, the glucocorticoid response element (GRE), a TGFβ-responsive AP-1 site, and two primary transcriptional elements requisite for basal transcription, the OC box and the TATA box, are indicated. The positioned nucleosome and DNase I-hypersensitive sites that are present when the gene is transcribed are also indicated. The Cbfa core recognition sequence at each site is indicated in boldface, with mutant nucleotides (mt) designated below. The lower panel shows the WT oligonucleotide probes, used in gel mobility shift assays, containing the site A, B, and C Cbfa motifs within the context of flanking sequences of the rat OC promoter.
FIG. 2
Mutations of the three rat OC Cbfa sites result in loss of Cbfa binding. (A) Formation of the osteoblast-specific complex from nuclear extracts of ROS 17/2.8 cells compared with oligonucleotides containing WT sequences representing Cbfa site A (lane 1), site B (lane 3), and site C (lane 5) and mutated (M) Cbfa sequences of sites A (lane 2), B (lane 4), and C (lane 6) in gel mobility shift assays. Cbfa complexes are indicated by solid arrowheads. A nonspecific complex (site B) is indicated by an open arrowhead. (B) Site A, site B, and site C show corresponding competition assays for each Cbfa site with the WT sequence as probe with increasing amounts (0, 20, 60, and 80×) of either WT oligonucleotide (lanes 1 to 4, respectively) or Cbfa site-mutated oligonucleotides (lanes 5 to 8, respectively) as the competitor.
FIG. 3
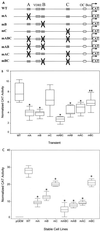
Requirement for multiple Cbfa sites for maximal transcriptional activity of the rat OC gene promoter. (A) Rat OC 5′ sequences (−1.1 kb) containing either single or multiple site mutations are schematically illustrated. Single-site mutations are designated mA, mB, or mC; two-site mutations are designated mAB, mAC, or mBC; and mABC is the triple mutation. (B) Following transient transfections in ROS 17/2.8 cells, normalized CAT reporter activity of the WT promoter and promoters with Cbfa site mutations are compared. Reporter activity was assayed 24 h after transfection of ROS 17/2.8 cells. The [14C]CAT activity was quantitated by a Betascope analyzer (Betagen, Waltham, Mass.) and normalized to that of luciferase. (Aliquots of the lysate were assayed for luciferase activity to normalize CAT activity). Each bar represents the LS mean ± standard error of the mean (SEM) (n = 12). (C) Activity of WT and Cbfa site-mutated promoters stably integrated into ROS 17/2.8 cells. Each group represents promoter activity in four independent cell lines, with each cell line assayed in four separate experiments in triplicate. The cells were harvested 3 days after being plated as the cells reached monolayer confluency for quantitation of CAT activity (normalized to total protein of the cell lysate). Each bar represents the LS mean ± SEM (n = 18). The pGEM control represents a promoterless CAT-containing stable cell line. Single asterisks, statistically less than WT, P < 0.001; double asterisks, P < 0.01.
FIG. 4
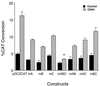
Cbfa1-mediated transactivation of OC promoters containing mutated Cbfa sites in HeLa cells. Cbfa1 expression plasmid (0.5 μg) and indicated OC-CAT plasmids (2.5 μg) were cotransfected into HeLa cells (n = 9 for each sample) and assayed 36 h following transfection. The CAT activity was quantitated by direct counting with a Betascope analyzer (Betagen). CAT activity was calculated as percent conversion and normalized for luciferase values used as internal controls. A statistically significant difference (P < 0.001) of the mABC group from WT is indicated by an asterisk.
FIG. 5
Cbfa sites regulate vitamin D-mediated transcription of the OC gene. (A) Loss of vitamin D (Vit-D) responsiveness of the rat OC promoter with three mutated Cbfa sites (mABC). Four independent ROS 17/2.8 cell lines with stably integrated WT promoters (WT1, WT2, WT3, and WT4) or with the triple Cbfa site mutation (mABC) (T1, T2, T3, and T4) were treated 3 days after being plated for 24 h with 10−8 M 1,25(OH)2D3 and assayed for CAT activity normalized to total protein in the cell lysate. Each bar represents the mean value of three determinations. (B) Distal Cbfa sites A and B in the OC promoter are required for vitamin D-induced transcriptional activity. ROS 17/2.8 cell lines containing stably integrated WT and the indicated Cbfa mutant promoter-CAT reporter constructs were examined for responsiveness to vitamin D (10−8 M 1,25(OH)2D3; 24 h). pGEM control is a promoterless-CAT stable cell line. The effect of vitamin D (vitamin D-treated/control untreated cells) is reported as fold induction. Each bar represents pooled data from three or four separate cell lines, each assayed in triplicate in two to four independent experiments. Asterisk, P < 0.001 (statistical significance of mutant cell line versus WT). The error bars indicate SD.
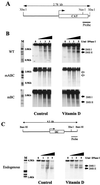
FIG. 6
DNase I-hypersensitive profile of WT and mutated OC promoters. (A) Diagrammatic illustration of the OC promoter-CAT transgene showing the region used as a probe. (B) Nuclei were isolated from untreated (left-hand panels) and vitamin D-treated [10−8 M 1,25(OH)2D3 for 24 h) (right-hand panels) ROS 17/2.8 stable cell lines having the 1.1-kb rat WT, mABC, or mBC OC promoter. Nuclei were incubated with increasing amounts of DNase I (from 0 to 5 U per 20 optical density at 260 μm units of nuclei) for 10 min at room temperature. The DNase I concentrations (U/ml) are designated above the lanes of the Southern blot. The purified DNA was digested with _Xba_I to detect the transgene. (C) DNase I hypersensitivity of the endogenous OC promoter from the mABC stable cell line. The _Bam_HI-_Xba_I fragment of the OC gene used as a probe is shown above. All samples (10 μg) were fractionated electrophoretically in a 1.2% agarose gel, and the blots were hybridized with the corresponding probe. Lane M, markers from λ DNA digested with _Hin_dIII and _Eco_RI. The two DNase I-hypersensitive sites (DHS I and DHS II) are indicated by solid arrowheads.
FIG. 7
Illustration of modifications in chromatin organization of the rat OC promoter. The top line diagrams random distribution of nucleosomes across −1.7 kb of the rat OC promoter in cells that do not express OC, as established by micrococcal nuclease digestion (46). The second line diagrams the actively transcribed OC promoter, showing the span of two DNase I-hypersensitive (DHS) sites together with regulatory elements in the proximal and distal DHS sites. The extent of DNase I hypersensitivity is compared for basal OC expression (WT-basal) and vitamin D-treated (WT–vitamin D-enhanced) osteoblasts (ROS 17/2.8) cells. No DHS, undetectable DNase I hypersensitivity in nonosseous cells or undetectable mABC OC promoter in osteoblasts.
Similar articles
- Basal and vitamin D-responsive activity of the rat osteocalcin promoter in stably transfected osteosarcoma cells: requirement of upstream sequences for control by the proximal regulatory domain.
Frenkel B, Montecino M, Green J, Aslam F, Desai R, Banerjee C, Stein JL, Lian JB, Stein GS. Frenkel B, et al. Endocrinology. 1996 Mar;137(3):1080-88. doi: 10.1210/endo.137.3.8603577. Endocrinology. 1996. PMID: 8603577 - The vitamin D response element in the distal osteocalcin promoter contributes to chromatin organization of the proximal regulatory domain.
Gutierrez S, Liu J, Javed A, Montecino M, Stein GS, Lian JB, Stein JL. Gutierrez S, et al. J Biol Chem. 2004 Oct 15;279(42):43581-8. doi: 10.1074/jbc.M408335200. Epub 2004 Aug 5. J Biol Chem. 2004. PMID: 15299011 - Regulation of the bone-specific osteocalcin gene by p300 requires Runx2/Cbfa1 and the vitamin D3 receptor but not p300 intrinsic histone acetyltransferase activity.
Sierra J, Villagra A, Paredes R, Cruzat F, Gutierrez S, Javed A, Arriagada G, Olate J, Imschenetzky M, Van Wijnen AJ, Lian JB, Stein GS, Stein JL, Montecino M. Sierra J, et al. Mol Cell Biol. 2003 May;23(9):3339-51. doi: 10.1128/MCB.23.9.3339-3351.2003. Mol Cell Biol. 2003. PMID: 12697832 Free PMC article. - Contributions of nuclear architecture and chromatin to vitamin D-dependent transcriptional control of the rat osteocalcin gene.
Lian JB, Stein JL, Stein GS, Montecino M, van Wijnen AJ, Javed A, Gutierrez S. Lian JB, et al. Steroids. 2001 Mar-May;66(3-5):159-70. doi: 10.1016/s0039-128x(00)00160-4. Steroids. 2001. PMID: 11179723 Review. - Nuclear structure--skeletal gene expression interrelationships.
Stein GS, van Wijnen AJ, Stein JL, Lian JB. Stein GS, et al. Front Biosci. 1998 Aug 1;3:d849-64. doi: 10.2741/a328. Front Biosci. 1998. PMID: 9682039 Review.
Cited by
- Integration of Runx and Smad regulatory signals at transcriptionally active subnuclear sites.
Zaidi SK, Sullivan AJ, van Wijnen AJ, Stein JL, Stein GS, Lian JB. Zaidi SK, et al. Proc Natl Acad Sci U S A. 2002 Jun 11;99(12):8048-53. doi: 10.1073/pnas.112664499. Proc Natl Acad Sci U S A. 2002. PMID: 12060751 Free PMC article. - runt homology domain transcription factors (Runx, Cbfa, and AML) mediate repression of the bone sialoprotein promoter: evidence for promoter context-dependent activity of Cbfa proteins.
Javed A, Barnes GL, Jasanya BO, Stein JL, Gerstenfeld L, Lian JB, Stein GS. Javed A, et al. Mol Cell Biol. 2001 Apr;21(8):2891-905. doi: 10.1128/MCB.21.8.2891-2905.2001. Mol Cell Biol. 2001. PMID: 11283267 Free PMC article. - Genetic and epigenetic regulation in nuclear microenvironments for biological control in cancer.
Stein GS, Zaidi SK, Stein JL, Lian JB, van Wijnen AJ, Montecino M, Young DW, Javed A, Pratap J, Choi JY, Ali SA, Pande S, Hassan MQ. Stein GS, et al. J Cell Biochem. 2008 Aug 15;104(6):2016-26. doi: 10.1002/jcb.21813. J Cell Biochem. 2008. PMID: 18615590 Free PMC article. Review. - Increased trabecular bone and improved biomechanics in an osteocalcin-null rat model created by CRISPR/Cas9 technology.
Lambert LJ, Challa AK, Niu A, Zhou L, Tucholski J, Johnson MS, Nagy TR, Eberhardt AW, Estep PN, Kesterson RA, Grams JM. Lambert LJ, et al. Dis Model Mech. 2016 Oct 1;9(10):1169-1179. doi: 10.1242/dmm.025247. Epub 2016 Jul 28. Dis Model Mech. 2016. PMID: 27483347 Free PMC article. - In vivo analysis of a developmental circuit for direct transcriptional activation and repression in the same cell by a Runx protein.
Canon J, Banerjee U. Canon J, et al. Genes Dev. 2003 Apr 1;17(7):838-43. doi: 10.1101/gad.1064803. Genes Dev. 2003. PMID: 12670867 Free PMC article.
References
- Ahn M Y, Bae S C, Maruyama M, Ito Y. Comparison of the human genomic structure of the runt domain-encoding PEBP2/CBFα gene family. Gene. 1996;168:279–280. - PubMed
- Archer T K, Lefebvre P, Wolford R G, Hager G L. Transcription factor loading on the MMTV promoter: a bimodal mechanism for promoter activation. Science. 1992;255:1573–1576. - PubMed
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1997.
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR039588/AR/NIAMS NIH HHS/United States
- R56 DE012528/DE/NIDCR NIH HHS/United States
- DE12528/DE/NIDCR NIH HHS/United States
- R37 DE012528/DE/NIDCR NIH HHS/United States
- AR39588/AR/NIAMS NIH HHS/United States
- R01 DE012528/DE/NIDCR NIH HHS/United States
- R03 TW000990/TW/FIC NIH HHS/United States
- AR56689/AR/NIAMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical