A human TATA binding protein-related protein with altered DNA binding specificity inhibits transcription from multiple promoters and activators - PubMed (original) (raw)
Comparative Study
A human TATA binding protein-related protein with altered DNA binding specificity inhibits transcription from multiple promoters and activators
P A Moore et al. Mol Cell Biol. 1999 Nov.
Abstract
The TATA binding protein (TBP) plays a central role in eukaryotic and archael transcription initiation. We describe the isolation of a novel 23-kDa human protein that displays 41% identity to TBP and is expressed in most human tissue. Recombinant TBP-related protein (TRP) displayed barely detectable binding to consensus TATA box sequences but bound with slightly higher affinities to nonconsensus TATA sequences. TRP did not substitute for TBP in transcription reactions in vitro. However, addition of TRP potently inhibited basal and activated transcription from multiple promoters in vitro and in vivo. General transcription factors TFIIA and TFIIB bound glutathione S-transferase-TRP in solution but failed to stimulate TRP binding to DNA. Preincubation of TRP with TFIIA inhibited TBP-TFIIA-DNA complex formation and addition of TFIIA overcame TRP-mediated transcription repression. TRP transcriptional repression activity was specifically reduced by mutations in TRP that disrupt the TFIIA binding surface but not by mutations that disrupt the TFIIB or DNA binding surface of TRP. These results suggest that TFIIA is a primary target of TRP transcription inhibition and that TRP may modulate transcription by a novel mechanism involving the partial mimicry of TBP functions.
Figures
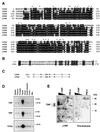
FIG. 1
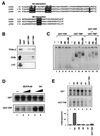
TRP sequence analysis and tissue distribution. (A) Alignment of the human TRP amino acid sequence with human (h), Drosophila (d), and yeast (y) TBP and Drosophila TRF1. White letters on a black background indicate amino acid identity. Black letters on a gray background indicate conservative changes in sequence. Asterisks indicate amino acid residues that make TBP DNA base contacts. (B) Alignment of the direct repeat sequence in human TRP. (C) Alignment of basic amino acid repeat regions of human TBP and TRP. (D) Tissue distribution of TRP expression determined by Northern blot analysis. Total RNA (2 μg) from various tissues was probed with 32P-labeled TRP (top gel), TBP (middle gel), or _TFIIA_γ (lower gel). The 1.35- and 2.4-kb RNA markers are indicated to the right. PBL, peripheral blood leukocytes. (E) TRP protein is made in HeLa cells. Polyclonal antibody directed against TRP (αTRP) or preimmune control serum was used to probe Western blots of HeLa cell nuclear extract (250 μg) or recombinant TRP (rTRP) (50 to 12 ng).
FIG. 2
Evolutionary relationships of the TBP family. (A) Percentages of similarity of TBP family members. (B) Phylogenetic tree of TBP and TRPs from various organisms. Numbers at the bottom represent percentages of disimilarity (i.e., TRP is 57.8% different than the other TBP molecules). Dros., Drosophila; S. pombe, Schizosaccharomyces pombe.
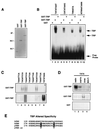
FIG. 3
TRP lacks TATA binding activity. (A) Purified GST, GST-TRP, and GST-TBP were visualized by Coomassie staining of SDS-polyacrylamide gels. Molecular masses (in kilodaltons) are noted at the left. (B) GST, GST-TBP, and GST-TRP were incubated with the radiolabeled Ad E1B promoter TATA element (TATATAAT), the HIV long terminal repeat promoter TATA element (CATATAAG), an oligonucleotide with two degenerate nucleotides (TNNATA), or an oligonucleotide with five degenerate nucleotides (NTNNATANN). DNAs bound by GST-TBP and GST-TRP are indicated by the arrows at the right. (C) EMSA indicating the GST-TRP (top gel)- or GST-TBP (bottom gel)-bound fraction of oligonucleotides containing the TATA-related sequences as depicted above each lane. (D) EMSA indicating the GST-TBP-, GST-TRP-, or GST-bound fraction of oligonucleotides containing TATA box sequences derived from the AdE4 (TATATA), HSP70 (TATAAA), SV40 (TATTTA), or EBV BZLF1 (TTTAAA) promoter. (E) TRP contains amino acid changes at positions known to alter DNA binding specificities of human and yeast TBP. as, altered specificities.
FIG. 4
TRP is a potent general transcription inhibitor in vitro. (A) TRP does not substitute for TBP. Nuclear extracts heat treated (Δ) to inactivate endogenous TBP were reconstituted with GST (lane 3), GST-TRP (lane 4), GST-TBP (lane 5), or partially purified TFIID (lane 6). Reaction mixtures with untreated nuclear extracts (NE) are indicated (lane 1). Shown is basal transcription with a TATA/Inr-containing promoter (top gel) or transcription activated by Zta (bottom gel). (B) Transcription reaction mixtures reconstituted with the untreated nuclear extracts and the TATA/Inr promoter in the presence of 50 ng of GST (lane 2), GST-TRP (lane 3), and GST-TBP (lane 4). (C) Transcription reaction mixtures activated by GAL4-AH (lanes 2 to 4), GAL4-CTF (lanes 5 to 7), GAL4-VP16 (lanes 8 to 10), or Zta (lanes 12 to 14) were reconstituted with 50 ng of GST (lanes 2, 5, 8, and 12), GST-TBP (lanes 3, 6, 9, and 13), or GST-TRP (lanes 4, 7, 10, and 14). Primer extension products from the G5E4TCAT and Z7E4TCAT reporters are indicated by the arrows at the left and right, respectively.
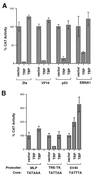
FIG. 5
TRP inhibits transcription from some promoters in HeLa cells. (A) HeLa cells were transfected with expression vectors for the Zta, GAL4-VP16, p53, and EBNA1 transcriptional activators and their respective CAT reporter plasmids and were cotransfected with the expression vector alone, TRP, or TBP (as indicated in the figure). (B) HeLa cells were transfected with CAT reporters containing the promoters derived from the Ad MLP, the TPA response element (TRE)-HSV TK, or the SV40 early promoter. CAT constructs were cotransfected with the expression vector control (vector), TRP, or a TBP expression plasmid. The sequences of promoter core TATA elements are indicated below the promoters. Values are the averages of results of three independent transfections and set as percentages of activated transcription.
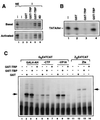
FIG. 6
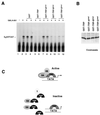
TFIIA can derepress TRP-mediated transcription inhibition. (A) Amino acid sequence alignment between TRP and human (h) and yeast (y) TBP residues known to interact with the general transcription factors TFIIA and TFIIB based on crystallographic and mutagenesis studies. (B) Interaction assay with purified GST, GST-TBP, and GST-TRP and in vitro-translated 35S-labeled TFIIAαβ (top gel), TFIIB (middle gel), or luciferase (LUC) (lower gel). Input represents 10% of the starting reaction mixture. (C) TRP inhibits TBP-TFIIA complex formation. The E1B TATA probe was preincubated with GST-TRP (lanes 2 to 5 and 10 to 13) and TFIIA (lanes 3, 7, and 11), TFIIB (lanes 4, 8, and 12), or both TFIIA and TFIIB (lanes 5, 9, and 13). GST-TBP (lanes 6 to 13) was added after a 15-min preincubation of other components and then incubated for an additional 30 min at 30°C. Bound complexes were analyzed by EMSA. (D) Transcription reaction mixtures reconstituted with HeLa nuclear extract, the GAL4-AH activator, and the G5E4TCAT template were incubated with 50 ng of GST control protein (top gel) or GST-TRP (lower gel). Transcription reaction mixtures were then supplemented with one or two transcription units of the TFIIE-TFIIF-polymerase II (PolII) fraction (lanes 2 and 3) or TFIIA (lanes 4 and 5). (E) Transcription reaction mixtures reconstituted with HeLa nuclear extract, the GAL4-AH activator, and the G5E4TCAT template were incubated with GST (top gel) or GST-TRP (bottom gel). Reaction mixtures were then supplemented with two transcription units of recombinant TFIIA (40 ng) (lane 2), TFIIB (20 ng) (lane 3), TFIIE (50 ng) (lane 4), TFIIF (50 ng) (lane 5), or GST-TBP (40 ng) (lane 6). Transcription derepression is quantified in the graph as percent derepression.
FIG. 7
A TRP mutant fails to repress transcription. (A) Transcription reaction mixtures were reconstituted with HeLa nuclear extract and the G5E4TCAT template. The GAL4-AH activator was added to the reaction mixtures in lanes 2 to 12. Reaction mixtures were supplemented with 40 ng of GST (lanes 3 and 4), GST-TRP (lanes 5 and 6), GST-TRP-Amut (lanes 7 and 8), GST-TRP-Bmut (lane 9 and 10), or GST-TRP-Dmut (lanes 11 and 12). (B) SDS-polyacrylamide gel electrophoresis analysis of GST-TRP, GST-TRP-Amut, GST-TRP-Bmut, and GST-TRP-Dmut preparations stained with Coomassie brilliant blue. (C) Model depicting the sequestration of TFIIA and potentially other unidentified TBP-interacting proteins (?) by TRP away from the TBP-TATA complex. TRP fails to bind TATA DNA but can efficiently bind TFIIA in solution. High levels of TRP down regulate TBP-dependent transcription by making TFIIA limiting for transcription.
Similar articles
- Virtually unidirectional binding of TBP to the AdMLP TATA box within the quaternary complex with TFIIA and TFIIB.
Kays AR, Schepartz A. Kays AR, et al. Chem Biol. 2000 Aug;7(8):601-10. doi: 10.1016/s1074-5521(00)00009-0. Chem Biol. 2000. PMID: 11048951 - A severely defective TATA-binding protein-TFIIB interaction does not preclude transcriptional activation in vivo.
Lee M, Struhl K. Lee M, et al. Mol Cell Biol. 1997 Mar;17(3):1336-45. doi: 10.1128/MCB.17.3.1336. Mol Cell Biol. 1997. PMID: 9032260 Free PMC article. - A novel TBP-interacting zinc finger protein represses transcription by inhibiting the recruitment of TFIIA and TFIIB.
Kim M, Park CH, Lee MS, Carlson BA, Hatfield DL, Lee BJ. Kim M, et al. Biochem Biophys Res Commun. 2003 Jun 20;306(1):231-8. doi: 10.1016/s0006-291x(03)00939-2. Biochem Biophys Res Commun. 2003. PMID: 12788093 - Role of the TATA-box binding protein (TBP) and associated family members in transcription regulation.
Mishal R, Luna-Arias JP. Mishal R, et al. Gene. 2022 Jul 30;833:146581. doi: 10.1016/j.gene.2022.146581. Epub 2022 May 18. Gene. 2022. PMID: 35597524 Review. - X-ray crystallographic studies of eukaryotic transcription initiation factors.
Burley SK. Burley SK. Philos Trans R Soc Lond B Biol Sci. 1996 Apr 29;351(1339):483-9. doi: 10.1098/rstb.1996.0046. Philos Trans R Soc Lond B Biol Sci. 1996. PMID: 8735270 Review.
Cited by
- Two isoforms of Drosophila TRF2 are involved in embryonic development, premeiotic chromatin condensation, and proper differentiation of germ cells of both sexes.
Kopytova DV, Krasnov AN, Kopantceva MR, Nabirochkina EN, Nikolenko JV, Maksimenko O, Kurshakova MM, Lebedeva LA, Yerokhin MM, Simonova OB, Korochkin LI, Tora L, Georgiev PG, Georgieva SG. Kopytova DV, et al. Mol Cell Biol. 2006 Oct;26(20):7492-505. doi: 10.1128/MCB.00349-06. Mol Cell Biol. 2006. PMID: 17015475 Free PMC article. - TATA-binding protein (TBP)-like factor (TLF) is a functional regulator of transcription: reciprocal regulation of the neurofibromatosis type 1 and c-fos genes by TLF/TRF2 and TBP.
Chong JA, Moran MM, Teichmann M, Kaczmarek JS, Roeder R, Clapham DE. Chong JA, et al. Mol Cell Biol. 2005 Apr;25(7):2632-43. doi: 10.1128/MCB.25.7.2632-2643.2005. Mol Cell Biol. 2005. PMID: 15767669 Free PMC article. - TBP paralogs accommodate metazoan- and vertebrate-specific developmental gene regulation.
Jacobi UG, Akkers RC, Pierson ES, Weeks DL, Dagle JM, Veenstra GJ. Jacobi UG, et al. EMBO J. 2007 Sep 5;26(17):3900-9. doi: 10.1038/sj.emboj.7601822. Epub 2007 Aug 16. EMBO J. 2007. PMID: 17703192 Free PMC article. - Heterozygous disruption of the TATA-binding protein gene in DT40 cells causes reduced cdc25B phosphatase expression and delayed mitosis.
Um M, Yamauchi J, Kato S, Manley JL. Um M, et al. Mol Cell Biol. 2001 Apr;21(7):2435-48. doi: 10.1128/MCB.21.7.2435-2448.2001. Mol Cell Biol. 2001. PMID: 11259592 Free PMC article. - TBP-related factors: a paradigm of diversity in transcription initiation.
Akhtar W, Veenstra GJ. Akhtar W, et al. Cell Biosci. 2011 Jun 27;1(1):23. doi: 10.1186/2045-3701-1-23. Cell Biosci. 2011. PMID: 21711503 Free PMC article.
References
- Adams M D, Dubnick M, Kerlavage A R, Moreno J M, Kelley T R, Utterback T R, Nagle J W, Fields C, Venter J C. Sequence identification of 2,375 human brain genes. Nature. 1992;355:632–634. - PubMed
- Adams M D, Kelley J M, Gocayne J D, Dubnick M, Polymeropoulos M H, Xiao H, Merril C R, Wu A, Olde B, Moreno R F, Kerlavage A R, McCombie W R, Venter J C. Complementary DNA sequencing: expressed sequence tags and human genome project. Science. 1991;252:1651–1656. - PubMed
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
- Auble D T, Hansen K E, Mueller C G, Lane W S, Thorner J, Hahn S. Mot1, a global repressor of RNA polymerase II transcription, inhibits TBP binding to DNA by an ATP-dependent mechanism. Genes Dev. 1994;8:1920–1934. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources