Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation - PubMed (original) (raw)
Cooperation between Th1 and Th2 cells in a murine model of eosinophilic airway inflammation
D A Randolph et al. J Clin Invest. 1999 Oct.
Abstract
We have studied the actions of helper T lymphocyte-1 and -2 (Th1 and Th2) cells in an acute model of eosinophilic airway inflammation by infusing chicken ovalbumin-specific (OVA-specific) Th1 cells, Th2 cells, or both into unsensitized mice and challenging the mice with an OVA aerosol. OVA challenge after infusion of Th1 cells alone resulted in airway inflammation with lymphocytes and monocytes. Challenge after the infusion of Th2 cells alone resulted in minimal inflammation. In contrast, when Th1 and Th2 cells were transferred together, they cooperated to promote a robust eosinophil-predominant inflammatory response. Th1 cells alone were readily recruited to the airways after challenge, but in the absence of Th1 cells, Th2 cells did not accumulate in the airways. When transferred together, both Th1 and Th2 cells, as well as endogenous eosinophils, were effectively recruited. This recruitment was correlated with increased VCAM-1 expression in the medium- and large-sized vessels of the lung and could be inhibited by treating the mice with neutralizing antibodies to TNF-alpha or VCAM-1. These data indicate that Th2 cells require signals in addition to antigen for their effective recruitment to the airways. Th1 cells can provide these signals.
Figures
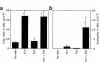
Figure 1
Synergy between Th1 and Th2 cells promotes eosinophilic airway inflammation. Here, 107 OVA-specific DO11.10 Th1 cells, 107 Th2 cells, or 107 of each were passively transferred to recipient mice by intravenous injection. The next day, in the morning and again in the afternoon, the mice were challenged with an aerosol of 1% OVA in sterile PBS. Three days after the challenge, the mice were sacrificed and BAL cells were collected. Shown is the average number of cells in each sample ± SD (n = 4 mice per group). (a) Total BAL nucleated cells. (b) BAL eosinophils. Similar results were obtained in 4 separate experiments. *Significantly different from all other groups (P < 0.05).
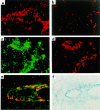
Figure 2
Th1 cells promote recruitment of Th2 cells to the airways after the challenge. Cultured T cells (107) were labeled with the fluorescent dyes PKH26 (red) or PKH67 (green) and transferred to recipient mice. In the morning and again in the afternoon of the next day, the mice were challenged with an aerosol of 1% OVA in sterile PBS. Three days after the challenge, lung tissue was collected and frozen. Fluorescent cells in the tissue were detected directly by fluorescence microscopy of air-dried 10-μm sections. Where indicated, eosinophils in the tissue were detected by virtue of the cyanide-resistant peroxidase activity in their granules. For detection of eosinophils, lung sections were fixed in acetone and then incubated in a DAB solution containing 1.6 mg/mL KCN. Shown are examples of lung tissue from recipients of (a) 107 PKH26-labeled Th1 cells, (b) 107 PKH26-labeled Th2 cells (c–f), 107 PKH67-labeled Th1 cells, and 107 PKH26-labeled Th2 cells. (c) Single exposure demonstrating Th1 cells. (d) Single exposure revealing Th2 cells in the same section as c. (e) Double exposure demonstrating Th1 and Th2 cells in close proximity to one another. (f) Detection of eosinophils (brown DAB staining) in the same section as e. Similar results were seen in 2 separate experiments.
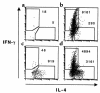
Figure 3
Flow cytometric analysis of Th1 and Th2 cell recruitment to the airways after challenge. Groups of 4 mice received (a) no transferred cells, (b) 107 DO11.10 Th1 cells, (c) 107 DO11.10 Th2 cells, or (d) 5 × 106 Th1 cells plus 5 × 106 Th2 cells. The mice were challenged, and BAL cells were collected as described in Figure 1. Cells from each group were pooled and then stimulated for 6 hours with PMA and ionomycin in the presence of monensin. Aliquots were stained with anti-CD4 and the anti-clonotypic antibody KJ1-26 to mark the transferred transgenic T cells. The cells were then fixed, permeabilized, and stained for intracellular IFN-γ and IL-4 as markers for Th1 and Th2 differentiation before analysis by flow cytometry. IFN-γ and IL-4 staining are shown for cells within the CD4+ KJ1-26+ gate. The numbers of cytokine-producing Th1 (IFN-γ+ IL-4–) and Th2 (IFN-γ– IL-4+) cells are indicated. Similar results were seen in 3 separate experiments.
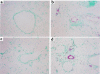
Figure 4
Th1 and Th2 cells cooperate to induce high levels of endothelial VCAM-1 expression and tissue eosinophilia. Mice received transferred T cells and were challenged as described in Figure 1. Two days after the challenge, the mice were sacrificed and lung tissue was collected and frozen. Sections of tissue were then cut and stained for the presence of VCAM-1 (red) and eosinophils (brown) as described in Methods. Shown are representative sections of lung from mice that received (a) no transferred cells, (b) Th1 cells, (c) Th2 cells, or (d) Th1 and Th2 cells. Similar results were seen in 4 separate experiments.
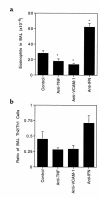
Figure 5
Neutralization of IFN-γ, TNF-α, or VCAM-1 alters eosinophil and Th2 cell recruitment. Mice received 107 Thy1.1 Th1 cells and 107 Thy1.2 Th2 cells by intravenous injection. On the following day, groups of 4 mice received injections of anti–glutathione-_S_-methyl transferase (control antibody), anti–TNF-α, anti–VCAM-1, or anti–IFN-γ antibodies and then were challenged with an aerosol of OVA as described in Figure 1. The day after the challenge, the anti-VCAM-1–treated group received an additional injection of antibody. Two days after the challenge, the mice were sacrificed and BAL cells were collected and counted. (a) The number of eosinophils in the BAL. Shown are the average values ± SEM for 2 pooled experiments. (b) The ratio of transferred Th2 to Th1 cells in the BAL. An aliquot of the BAL cells from each animal was stained for CD4, KJ1-26, and Thy1.2 and analyzed by flow cytometry. The average ratio of CD4+KJ1-26+Thy1.2+ to CD4+KJ1-26+Thy1.2– cells is shown. *Significantly different from control antibody–treated group (P < 0.05).
Figure 6
Neutralizing antibody against TNF-α inhibits VCAM-1 expression and tissue eosinophilia. Mice were treated as described in Figure 5. After BAL cells were harvested, the lung tissue was collected and frozen. Sections of lung were then stained for the presence of VCAM-1 (red staining) and eosinophils (brown staining). (a) Section from a mouse treated with anti–TNF-α, and (b) section from a mouse treated with anti–glutathione-_S_-methyl transferase (control antibody). Similar results were seen in 2 separate experiments.
Similar articles
- Modulation of airway inflammation by passive transfer of allergen-specific Th1 and Th2 cells in a mouse model of asthma.
Randolph DA, Carruthers CJ, Szabo SJ, Murphy KM, Chaplin DD. Randolph DA, et al. J Immunol. 1999 Feb 15;162(4):2375-83. J Immunol. 1999. PMID: 9973518 - Cell cooperation in development of eosinophil-predominant inflammation in airways.
Chaplin DD. Chaplin DD. Immunol Res. 2002;26(1-3):55-62. doi: 10.1385/IR:26:1-3:055. Immunol Res. 2002. PMID: 12403345 Review. - Toll-like receptor-2 agonist-allergen coupling efficiently redirects Th2 cell responses and inhibits allergic airway eosinophilia.
Krishnaswamy JK, Jirmo AC, Baru AM, Ebensen T, Guzmán CA, Sparwasser T, Behrens GM. Krishnaswamy JK, et al. Am J Respir Cell Mol Biol. 2012 Dec;47(6):852-63. doi: 10.1165/rcmb.2011-0414OC. Epub 2012 Sep 6. Am J Respir Cell Mol Biol. 2012. PMID: 22962064 - New production of eosinophils and the corresponding TH1/TH2 balance in the lungs after allergen exposure in BALB/c and C57BL/6 mice.
Lu Y, Sjöstrand M, Malmhäll C, Rådinger M, Jeurink P, Lötvall J, Bossios A. Lu Y, et al. Scand J Immunol. 2010 Mar;71(3):176-85. doi: 10.1111/j.1365-3083.2009.02363.x. Scand J Immunol. 2010. PMID: 20415783 - The role of nitric oxide in the development of asthma.
Curran AD. Curran AD. Int Arch Allergy Immunol. 1996 Sep;111(1):1-4. doi: 10.1159/000237336. Int Arch Allergy Immunol. 1996. PMID: 8753836 Review.
Cited by
- Both Th1 and Th2 cells require P-selectin glycoprotein ligand-1 for optimal rolling on inflamed endothelium.
Mangan PR, O'Quinn D, Harrington L, Bonder CS, Kubes P, Kucik DF, Bullard DC, Weaver CT. Mangan PR, et al. Am J Pathol. 2005 Dec;167(6):1661-75. doi: 10.1016/S0002-9440(10)61249-7. Am J Pathol. 2005. PMID: 16314478 Free PMC article. - MMP induced by Gr-1+ cells are crucial for recruitment of Th cells into the airways.
Jung YW, Zindl CL, Lai JF, Weaver CT, Chaplin DD. Jung YW, et al. Eur J Immunol. 2009 Aug;39(8):2281-92. doi: 10.1002/eji.200838985. Eur J Immunol. 2009. PMID: 19593770 Free PMC article. - The preventive effect of Brassica napus L. oil on pathophysiological changes of respiratory system in experimental asthmatic rat.
Kabiri Rad M, Neamati A, Boskabady MH, Mahdavi-Shahri N, Mahmoudabady M. Kabiri Rad M, et al. Avicenna J Phytomed. 2013 Winter;3(1):56-63. Avicenna J Phytomed. 2013. PMID: 25050259 Free PMC article. - Distinct spatial and temporal roles for Th1, Th2, and Th17 cells in asthma.
Luo W, Hu J, Xu W, Dong J. Luo W, et al. Front Immunol. 2022 Aug 12;13:974066. doi: 10.3389/fimmu.2022.974066. eCollection 2022. Front Immunol. 2022. PMID: 36032162 Free PMC article. Review. - IL-9 and IL-13 induce mucous cell metaplasia that is reduced by IFN-gamma in a Bax-mediated pathway.
Xiang J, Rir-Sim-Ah J, Tesfaigzi Y. Xiang J, et al. Am J Respir Cell Mol Biol. 2008 Mar;38(3):310-7. doi: 10.1165/rcmb.2007-0078OC. Epub 2007 Sep 27. Am J Respir Cell Mol Biol. 2008. PMID: 17901408 Free PMC article.
References
- Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. - PubMed
- Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. - PubMed
- Finkelman FD, et al. Lymphokine control of in vivo immunoglobulin isotype selection. Annu Rev Immunol. 1990;8:303–333. - PubMed
- Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 GM07200/GM/NIGMS NIH HHS/United States
- HL-56419/HL/NHLBI NIH HHS/United States
- P50 HL056419/HL/NHLBI NIH HHS/United States
- T32 GM007200/GM/NIGMS NIH HHS/United States
- AI-34580/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous