Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells - PubMed (original) (raw)
Differential expression of three T lymphocyte-activating CXC chemokines by human atheroma-associated cells
F Mach et al. J Clin Invest. 1999 Oct.
Abstract
Activated T lymphocytes accumulate early in atheroma formation and persist at sites of lesion growth and rupture, suggesting that they may play an important role in the pathogenesis of atherosclerosis. Moreover, atherosclerotic lesions contain the Th1-type cytokine IFN-gamma, a potentiator of atherosclerosis. The present study demonstrates the differential expression of the 3 IFN-gamma-inducible CXC chemokines--IFN-inducible protein 10 (IP-10), monokine induced by IFN-gamma (Mig), and IFN-inducible T-cell alpha chemoattractant (I-TAC)--by atheroma-associated cells, as well as the expression of their receptor, CXCR3, by all T lymphocytes within human atherosclerotic lesions in situ. Atheroma-associated endothelial cells (ECs), smooth muscle cells (SMCs), and macrophages (MO) all expressed IP-10, whereas Mig and I-TAC were mainly expressed in ECs and MO, as detected by double immunofluorescence staining. ECs of microvessels within lesions also expressed abundant I-TAC. In vitro experiments supported these results and showed that IL-1beta, TNF-alpha, and CD40 ligand potentiated IP-10 expression from IFN-gamma-stimulated ECs. In addition, nitric oxide (NO) treatment decreased IFN-gamma induction of IP-10. Our findings suggest that the differential expression of IP-10, Mig, and I-TAC by atheroma-associated cells plays a role in the recruitment and retention of activated T lymphocytes observed within vascular wall lesions during atherogenesis.
Figures
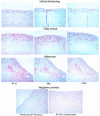
Figure 1
Expression of IP-10, Mig, and I-TAC in human atherosclerotic lesions in situ. Human carotid arteries sections were stained with specific antibodies to IP-10, Mig, and I-TAC. High-power view (×100) of atherosclerotic lesions from different stages (Intimal thickening, Fatty streak, and Atheroma) revealed the expression of the chemokines (red-brown reaction product). Adjacent sections of the same atherosclerotic carotid tissue stained for IP-10 was stained with the rabbit preimmune IP-10 serum (×100). Normal human artery exhibited no expression of IP-10 (×100). The lumen of the artery is at the upper left side of each photomicrograph. Analysis of 5–7 atheroma at each stage of lesion development from different donors, and normal tissue from 4 different donors showed similar results.
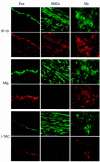
Figure 2
Colocalization of IP-10, Mig, and I-TAC with ECs, SMCs, and MØ in human atherosclerotic lesions. High-power views (×400) of human carotid sections showed specific staining for IP-10, Mig, and I-TAC (Texas red staining) within the atherosclerotic lesions. Cell types were characterized by immunofluorescence staining with anti-CD31 mAb for ECs, anti–α-actin mAb for SMCs, and anti-CD68 mAb for MØ (FITC; green staining). The lumen of the artery is at the top of each photomicrograph. Analysis of atheroma from 3 different donors showed similar results.
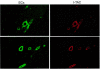
Figure 3
Expression of I-TAC by ECs of microvessels within human atherosclerotic lesions. High-power views (×400) of human carotid sections showed specific staining for I-TAC (Texas red staining). Cell types was characterized by immunofluorescence staining of CD31 for ECs within the atherosclerotic lesions (FITC; green staining). The lumen of the artery is at the top of each photomicrograph. Analysis of atheroma from 3 different donors showed similar results.
Figure 4
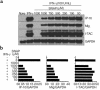
Western blot analysis of vascular tissue extracts. Samples (50 μg protein per lane) from normal human aortas (Normal) and human carotid atherosclerotic lesions (Plaque) were analyzed for IP-10 and Mig expression by Western blot. Analysis of 3 normal and 4 atherosclerotic tissues from different donors are shown. Human recombinant IP-10 (rIP-10) and Mig (rMig) were used as controls. The position of the molecular markers markers are indicated (kDa).
Figure 5
Expression of the chemokine-receptor CXCR3 on T lymphocytes within human atherosclerotic lesions. Adjacent sections of human carotid arteries at different stages (Intimal thickening, Fatty streak, and Atheroma) were stained with anti-CD3 or anti-CXCR3 antibody (arrows). Photomicrographs (×100) reveal CD3 and CXCR3 expression (red-brown reaction product). The lumen of the artery is at the top of each photomicrograph. Analysis of 5–7 atheroma at each stage of lesion development from different donors showed similar results.
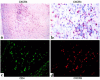
Figure 6
Expression of CXCR3 on CD4+ T lymphocytes within human atherosclerotic lesions. Sections of human carotid arteries were stained with anti-CXCR3 antibody. (a) Low-power (×100) and (b) high-power (×400) views show CXCR3 expression (red-brown reaction product) in the shoulder region of the plaque. Colocalization of CD4+ lymphocytes (c) (FITC; green staining) with CXCR3 (d) (Texas red staining) was performed by immunofluorescence-double staining. The lumen of the artery is at the top of each photomicrograph. Analysis of atheroma from 4 different donors showed similar results
Figure 7
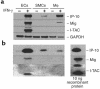
Expression of IP-10, Mig, and I-TAC by human ECs, SMCs, and monocyte-derived MØ in vitro. (a) Northern blot and (b) Western analysis of unstimulated (–) or IFN-γ−stimulated (1,000 U/mL for 18 hours) (+) ECs, SMCs, and monocyte-derived MØ was performed using 20 μg total RNA per lane and 45 μL of unconcentrated cell supernatant per lane, respectively. GAPDH was used as a control for RNA loading. Recombinant chemokines (10 ng per lane) were used for controls in Western blots. For Northern Blots, the IP-10 and I-TAC blots were exposed for 7 hours, whereas the Mig blot was exposed for 70 hours. Similar results were obtained in independent experiments with cells from 4 different donors.
Figure 8
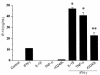
Secretion of IP-10 protein by human ECs in response to cytokine stimulation. Supernatants from human ECs unstimulated (–) or stimulated for 24 hours with IL-1β (10 ng/mL), TNF-α (50 ng/mL), IFN-γ (100 U/mL), or CD40L (5 μg/mL) were analyzed by ELISA for IP-10 levels. This figure is representative of 3 separate experiments using ECs from different donors. Error bars represent SD from triplicates (*P < 0.0001, **P < 0.005).
Figure 9
NO regulation of IFN-γ–induced IP-10, Mig, and I-TAC from human vascular ECs. (a) Northern blot analysis of unstimulated (None) or IFN-γ–stimulated (100 U/mL for 24 hours) ECs pretreated with various concentration of the NO donor SNAP. GAPDH was used as a control for RNA loading. The IP-10, Mig, and I-TAC blots were exposed for 6 hours, 24 hours, and 20 hours, respectively. (b) Quantification of Northern blots expressed as the ratio of chemokine/GAPDH signal for each sample using a phosphoimager to normalize for differences in RNA loading between samples on a given blot. Because of differences in exposure times used for the quantification, comparisons of chemokine expression can only be made within a given blot. Similar results were obtained in independent experiments with cells from 4 different donors.
Similar articles
- The T cell-specific CXC chemokines IP-10, Mig, and I-TAC are expressed by activated human bronchial epithelial cells.
Sauty A, Dziejman M, Taha RA, Iarossi AS, Neote K, Garcia-Zepeda EA, Hamid Q, Luster AD. Sauty A, et al. J Immunol. 1999 Mar 15;162(6):3549-58. J Immunol. 1999. PMID: 10092813 - Gene expression and production of the monokine induced by IFN-gamma (MIG), IFN-inducible T cell alpha chemoattractant (I-TAC), and IFN-gamma-inducible protein-10 (IP-10) chemokines by human neutrophils.
Gasperini S, Marchi M, Calzetti F, Laudanna C, Vicentini L, Olsen H, Murphy M, Liao F, Farber J, Cassatella MA. Gasperini S, et al. J Immunol. 1999 Apr 15;162(8):4928-37. J Immunol. 1999. PMID: 10202039 - Hypersensitivity pneumonitis and alpha-chemokines.
Limongi F, Fallahi P. Limongi F, et al. Clin Ter. 2017 Mar-Apr;168(2):e140-e145. doi: 10.7417/CT.2017.1996. Clin Ter. 2017. PMID: 28383627 Review. - Role for interferon-gamma inducible chemokines in endocrine autoimmunity: an expanding field.
Rotondi M, Lazzeri E, Romagnani P, Serio M. Rotondi M, et al. J Endocrinol Invest. 2003 Feb;26(2):177-80. doi: 10.1007/BF03345149. J Endocrinol Invest. 2003. PMID: 12739748 Review.
Cited by
- Distinctive expression of chemokines and transforming growth factor-beta signaling in human arterial endothelium during atherosclerosis.
Volger OL, Fledderus JO, Kisters N, Fontijn RD, Moerland PD, Kuiper J, van Berkel TJ, Bijnens AP, Daemen MJ, Pannekoek H, Horrevoets AJ. Volger OL, et al. Am J Pathol. 2007 Jul;171(1):326-37. doi: 10.2353/ajpath.2007.061196. Am J Pathol. 2007. PMID: 17591977 Free PMC article. - The production of CXCR3-agonistic chemokines by synovial fibroblasts from patients with rheumatoid arthritis.
Ueno A, Yamamura M, Iwahashi M, Okamoto A, Aita T, Ogawa N, Makino H. Ueno A, et al. Rheumatol Int. 2005 Jun;25(5):361-7. doi: 10.1007/s00296-004-0449-x. Epub 2004 Mar 5. Rheumatol Int. 2005. PMID: 15004722 - Molecular imaging in atherosclerosis.
Glaudemans AW, Slart RH, Bozzao A, Bonanno E, Arca M, Dierckx RA, Signore A. Glaudemans AW, et al. Eur J Nucl Med Mol Imaging. 2010 Dec;37(12):2381-97. doi: 10.1007/s00259-010-1406-4. Epub 2010 Mar 20. Eur J Nucl Med Mol Imaging. 2010. PMID: 20306036 Free PMC article. Review. - The Most Promising Biomarkers of Allogeneic Kidney Transplant Rejection.
Rogulska K, Wojciechowska-Koszko I, Dołęgowska B, Kwiatkowska E, Roszkowska P, Kapczuk P, Kosik-Bogacka D. Rogulska K, et al. J Immunol Res. 2022 May 28;2022:6572338. doi: 10.1155/2022/6572338. eCollection 2022. J Immunol Res. 2022. PMID: 35669103 Free PMC article. Review. - CXCL9 and its Receptor CXCR3, an Important Link Between Inflammation and Cardiovascular Risks in RA Patients.
Shamsi A, Roghani SA, Abdan Z, Soufivand P, Pournazari M, Bahrehmand F, Vafaei A, Salari N, Soroush MG, Taghadosi M. Shamsi A, et al. Inflammation. 2023 Dec;46(6):2374-2385. doi: 10.1007/s10753-023-01884-5. Epub 2023 Aug 5. Inflammation. 2023. PMID: 37542661
References
- Libby P. Molecular bases of the acute coronary syndromes. Circulation. 1995;91:2844–2850. - PubMed
- Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med. 1999;340:115–126. - PubMed
- Hansson GK, et al. Localization of T lymphocytes and macrophages in fibrous and complicated human atherosclerotic plaques. Atherosclerosis. 1988;72:135–141. - PubMed
- Hansson GK. Cell-mediated immunity in atherosclerosis. Curr Opin Lipidol. 1997;8:301–311. - PubMed
- Stemme S, Hansson GK. Immune mechanisms in atherogenesis. Ann Med. 1994;26:141–146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 HL034636/HL/NHLBI NIH HHS/United States
- R37 HL034636/HL/NHLBI NIH HHS/United States
- R01 CA069212/CA/NCI NIH HHS/United States
- HL-34636/HL/NHLBI NIH HHS/United States
- CA-69212/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials