Human cyclin A is required for mitosis until mid prophase - PubMed (original) (raw)
Human cyclin A is required for mitosis until mid prophase
N Furuno et al. J Cell Biol. 1999.
Abstract
We have used microinjection and time-lapse video microscopy to study the role of cyclin A in mitosis. We have injected purified, active cyclin A/cyclin-dependent kinase 2 (CDK2) into synchronized cells at specific points in the cell cycle and assayed its effect on cell division. We find that cyclin A/CDK2 will drive G2 phase cells into mitosis within 30 min of microinjection, up to 4 h before control cells enter mitosis. Often this premature mitosis is abnormal; the chromosomes do not completely condense and daughter cells fuse. Remarkably, microinjecting cyclin A/CDK2 into S phase cells has no effect on progress through the following G2 phase or mitosis. In complementary experiments we have microinjected the amino terminus of p21(Cip1/Waf1/Sdi1) (p21N) into cells to inhibit cyclin A/CDK2 activity. We find that p21N will prevent S phase or G2 phase cells from entering mitosis, and will cause early prophase cells to return to interphase. These results suggest that cyclin A/CDK2 is a rate-limiting component required for entry into mitosis, and for progress through mitosis until late prophase. They also suggest that cyclin A/CDK2 may be the target of the recently described prophase checkpoint.
Figures
Figure 1
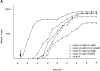
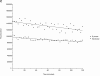
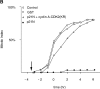
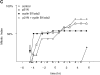
(A) Cyclin A/CDK2 will promote mitosis in G2 phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 7 h cells entered G2 phase and at least 35 cells were injected (time marked by the arrow) with either cyclin A/CDK2 (2–4 μM, filled diamonds; 0.4–0.8 μM, open diamonds), or cyclin E-CDK2 (2–4 μM, filled squares), or cyclin A/CDK2K33R (open squares) or uninjected (open circles). Cells were followed by time-lapse DIC microscopy to determine when they entered mitosis as defined by rounding up and chromosome condensation. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least two independent experiments. (B) Cyclin A/CDK2 does not promote mitosis in S phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 2 h cells were either injected (time marked by the arrow) with cyclin A/CDK2 (2–4 μM; filled diamonds, n = 36) or not (open circles) and followed by time-lapse microscopy to determine when they entered mitosis. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least three independent experiments. (C) Cyclin A/CDK2 is not inactivated in S phase cells by proteolysis. HeLa cells were synchronized at the beginning of S phase or G2 phase by a thymidine-aphidicolin block and release regime. S phase (squares) or G2 phase (diamonds) cells were injected with purified cyclin A-GFP and imaged by DIC and epifluorescence microscopy every 3 min, keeping all parameters (exposure time, magnification and numerical aperture of the lens) constant. The amount of fluorescence in the cells was quantified (see Materials and Methods) and the rate of decrease calculated for S phase (114 pixels per minute) and G2 phase (224 pixels per minute). The effect of bleaching was minimal as most of the decrease was prevented by adding a proteasome inhibitor MG132 (not shown). Values are representative of two independent experiments.
Figure 1
(A) Cyclin A/CDK2 will promote mitosis in G2 phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 7 h cells entered G2 phase and at least 35 cells were injected (time marked by the arrow) with either cyclin A/CDK2 (2–4 μM, filled diamonds; 0.4–0.8 μM, open diamonds), or cyclin E-CDK2 (2–4 μM, filled squares), or cyclin A/CDK2K33R (open squares) or uninjected (open circles). Cells were followed by time-lapse DIC microscopy to determine when they entered mitosis as defined by rounding up and chromosome condensation. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least two independent experiments. (B) Cyclin A/CDK2 does not promote mitosis in S phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 2 h cells were either injected (time marked by the arrow) with cyclin A/CDK2 (2–4 μM; filled diamonds, n = 36) or not (open circles) and followed by time-lapse microscopy to determine when they entered mitosis. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least three independent experiments. (C) Cyclin A/CDK2 is not inactivated in S phase cells by proteolysis. HeLa cells were synchronized at the beginning of S phase or G2 phase by a thymidine-aphidicolin block and release regime. S phase (squares) or G2 phase (diamonds) cells were injected with purified cyclin A-GFP and imaged by DIC and epifluorescence microscopy every 3 min, keeping all parameters (exposure time, magnification and numerical aperture of the lens) constant. The amount of fluorescence in the cells was quantified (see Materials and Methods) and the rate of decrease calculated for S phase (114 pixels per minute) and G2 phase (224 pixels per minute). The effect of bleaching was minimal as most of the decrease was prevented by adding a proteasome inhibitor MG132 (not shown). Values are representative of two independent experiments.
Figure 1
(A) Cyclin A/CDK2 will promote mitosis in G2 phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 7 h cells entered G2 phase and at least 35 cells were injected (time marked by the arrow) with either cyclin A/CDK2 (2–4 μM, filled diamonds; 0.4–0.8 μM, open diamonds), or cyclin E-CDK2 (2–4 μM, filled squares), or cyclin A/CDK2K33R (open squares) or uninjected (open circles). Cells were followed by time-lapse DIC microscopy to determine when they entered mitosis as defined by rounding up and chromosome condensation. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least two independent experiments. (B) Cyclin A/CDK2 does not promote mitosis in S phase cells. HeLa cells were synchronized at the beginning of S phase by a thymidine-aphidicolin block and then released. After 2 h cells were either injected (time marked by the arrow) with cyclin A/CDK2 (2–4 μM; filled diamonds, n = 36) or not (open circles) and followed by time-lapse microscopy to determine when they entered mitosis. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least three independent experiments. (C) Cyclin A/CDK2 is not inactivated in S phase cells by proteolysis. HeLa cells were synchronized at the beginning of S phase or G2 phase by a thymidine-aphidicolin block and release regime. S phase (squares) or G2 phase (diamonds) cells were injected with purified cyclin A-GFP and imaged by DIC and epifluorescence microscopy every 3 min, keeping all parameters (exposure time, magnification and numerical aperture of the lens) constant. The amount of fluorescence in the cells was quantified (see Materials and Methods) and the rate of decrease calculated for S phase (114 pixels per minute) and G2 phase (224 pixels per minute). The effect of bleaching was minimal as most of the decrease was prevented by adding a proteasome inhibitor MG132 (not shown). Values are representative of two independent experiments.
Figure 2
Cells forced into premature mitosis by cyclin A often fail to condense fully their chromosomes. HeLa cells were synchronized in G2 phase and injected with 2–4 μM cyclin A/CDK2 as in Fig. 1 A. Representative DIC images are shown of a cell that does (A) and a cell that does not (B) fully condense its chromosomes at metaphase. The cell that does not condense its chromosomes divides but the daughter cells fuse.
Figure 3
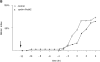
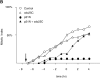
A catalytically inactive mutant of Cdc25B prevents cyclin A/CDK2 from promoting premature mitosis. HeLa cells in S phase were microinjected or not with a catalytically inactive form of cdc25B3 cDNA under the CMV promoter at a concentration of 0.1 μg/μl. When cells entered G2 phase (∼4 h later) they were injected or not with cyclin A/CDK2 to a final concentration of ∼2–4 μM. A minimum of 36 cells for each sample were followed by time-lapse DIC microscopy as in Fig. 1 and the results are plotted with zero time set as the time when 50% of the control cells entered mitosis. The results are representative of at least two independent experiments.
Figure 4
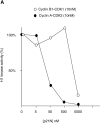
p21N inhibits cyclin A/CDK2 and blocks cells in G2 phase. (A) p21N inhibits cyclin A/CDK2 better than cyclin B-cdc2 in vitro. Cyclin A/CDK2 and cyclin B-cdc2 were produced and purified from baculovirus infected cells and assayed for histone H1 kinase activity with γ-[33P]ATP. GST-p21N was produced and purified from E. coli and added to the cyclin-Cdk incubations at the indicated concentrations. At a 40-fold excess of p21N, cyclin A/CDK2 is almost completely inhibited but cyclin B1-CDK1 is unaffected. (B) p21N prevents cells from entering mitosis. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N (filled circles, representing ∼40-fold excess over cyclin A/CDK2), or p21N preincubated with cyclin A/CDK2K33R (open diamonds), or with GST (open squares) and their entry into mitosis followed by time-lapse microscopy. A minimum of 25 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) Cells arrested by p21N arrest in G2 phase. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N and fixed and stained for immunofluorescence. Cells were stained with anti–cyclin B1 as a marker of G2 phase, and anti-tubulin to visualize any mitotic spindles. (a) Control cells that have mostly reentered G1 phase; (b) p21N-injected cells fixed at a time when all the control cells had completed mitosis.
Figure 4
p21N inhibits cyclin A/CDK2 and blocks cells in G2 phase. (A) p21N inhibits cyclin A/CDK2 better than cyclin B-cdc2 in vitro. Cyclin A/CDK2 and cyclin B-cdc2 were produced and purified from baculovirus infected cells and assayed for histone H1 kinase activity with γ-[33P]ATP. GST-p21N was produced and purified from E. coli and added to the cyclin-Cdk incubations at the indicated concentrations. At a 40-fold excess of p21N, cyclin A/CDK2 is almost completely inhibited but cyclin B1-CDK1 is unaffected. (B) p21N prevents cells from entering mitosis. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N (filled circles, representing ∼40-fold excess over cyclin A/CDK2), or p21N preincubated with cyclin A/CDK2K33R (open diamonds), or with GST (open squares) and their entry into mitosis followed by time-lapse microscopy. A minimum of 25 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) Cells arrested by p21N arrest in G2 phase. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N and fixed and stained for immunofluorescence. Cells were stained with anti–cyclin B1 as a marker of G2 phase, and anti-tubulin to visualize any mitotic spindles. (a) Control cells that have mostly reentered G1 phase; (b) p21N-injected cells fixed at a time when all the control cells had completed mitosis.
Figure 4
p21N inhibits cyclin A/CDK2 and blocks cells in G2 phase. (A) p21N inhibits cyclin A/CDK2 better than cyclin B-cdc2 in vitro. Cyclin A/CDK2 and cyclin B-cdc2 were produced and purified from baculovirus infected cells and assayed for histone H1 kinase activity with γ-[33P]ATP. GST-p21N was produced and purified from E. coli and added to the cyclin-Cdk incubations at the indicated concentrations. At a 40-fold excess of p21N, cyclin A/CDK2 is almost completely inhibited but cyclin B1-CDK1 is unaffected. (B) p21N prevents cells from entering mitosis. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N (filled circles, representing ∼40-fold excess over cyclin A/CDK2), or p21N preincubated with cyclin A/CDK2K33R (open diamonds), or with GST (open squares) and their entry into mitosis followed by time-lapse microscopy. A minimum of 25 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) Cells arrested by p21N arrest in G2 phase. G2 phase HeLa cells were injected or not with a final concentration of 2 μM p21N and fixed and stained for immunofluorescence. Cells were stained with anti–cyclin B1 as a marker of G2 phase, and anti-tubulin to visualize any mitotic spindles. (a) Control cells that have mostly reentered G1 phase; (b) p21N-injected cells fixed at a time when all the control cells had completed mitosis.
Figure 5
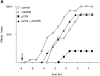
Activators of cyclin B-CDK2 and active cyclin B-CDK2 itself overcome the p21 block. (A) G2 phase HeLa cells were microinjected or not at the indicated time with p21 to a final concentration of 2–4 μM, and the cDNA encoding cdc25B3 under the CMV promoter at a concentration of 0.1μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 21 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (B) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and the cDNA encoding cdc25C under the CMV promoter at a concentration of 0.1 μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 23 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and purified active cyclin B1-CDK1 (∼2 μM). Entry into mitosis was followed by time-lapse microscopy and a minimum of 20 cells was followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments.
Figure 5
Activators of cyclin B-CDK2 and active cyclin B-CDK2 itself overcome the p21 block. (A) G2 phase HeLa cells were microinjected or not at the indicated time with p21 to a final concentration of 2–4 μM, and the cDNA encoding cdc25B3 under the CMV promoter at a concentration of 0.1μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 21 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (B) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and the cDNA encoding cdc25C under the CMV promoter at a concentration of 0.1 μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 23 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and purified active cyclin B1-CDK1 (∼2 μM). Entry into mitosis was followed by time-lapse microscopy and a minimum of 20 cells was followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments.
Figure 5
Activators of cyclin B-CDK2 and active cyclin B-CDK2 itself overcome the p21 block. (A) G2 phase HeLa cells were microinjected or not at the indicated time with p21 to a final concentration of 2–4 μM, and the cDNA encoding cdc25B3 under the CMV promoter at a concentration of 0.1μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 21 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (B) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and the cDNA encoding cdc25C under the CMV promoter at a concentration of 0.1 μg/μl. Entry into mitosis was followed by time-lapse microscopy and a minimum of 23 cells were followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments. (C) G2 phase HeLa cells were microinjected or not at the indicated time with p21N to a final concentration of 2–4 μM, and purified active cyclin B1-CDK1 (∼2 μM). Entry into mitosis was followed by time-lapse microscopy and a minimum of 20 cells was followed for each sample. The results are plotted with zero time set as the time when 50% of the control cells entered mitosis and are representative of at least two independent experiments.
Figure 6
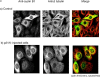
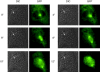
p21N will cause a prophase cell to return to G2 phase. PtK1 cells in prophase were microinjected with purified GST-p21N to a final concentration of 2–4 μM and visualized by time-lapse DIC microscopy. (A) PtK1 cells just before microinjection at 13.20. The condensed chromosomes are clearly visible. (B) The same PtK1 cell 115 min after injection of p21N. The chromosomes have decondensed and the nucleolus has reformed. Images are representative of 17 cells in at least five independent experiments.
Figure 7
Cyclin B1-GFP translocates into the nucleus at the end of prophase just after the nucleolus disappears. PtK1 cells in late G2 phase were injected with purified cyclin B1-GFP. Cells were imaged by time-lapse fluorescence and DIC microscopy and images captured at the indicated times. DIC and fluorescence images show that cyclin B1-GFP translocates into the nucleus just after the nucleolus disappears between the 8″ and 10″ time points (the nucleolus is indicated by an arrow). Images are representative of two independent experiments in this study, and confirmed by several other studies in this laboratory (Hagting et al. 1999).
Similar articles
- Cdk2 activity is dispensable for the onset of DNA replication during the first mitotic cycles of the sea urchin early embryo.
Moreau JL, Marques F, Barakat A, Schatt P, Lozano JC, Peaucellier G, Picard A, Genevière AM. Moreau JL, et al. Dev Biol. 1998 Aug 15;200(2):182-97. doi: 10.1006/dbio.1998.8961. Dev Biol. 1998. PMID: 9705226 - Role for cyclin-dependent kinase 2 in mitosis exit.
D'Angiolella V, Costanzo V, Gottesman ME, Avvedimento EV, Gautier J, Grieco D. D'Angiolella V, et al. Curr Biol. 2001 Aug 7;11(15):1221-6. doi: 10.1016/s0960-9822(01)00352-9. Curr Biol. 2001. PMID: 11516956 - Phosphorylation of Mcm4 at specific sites by cyclin-dependent kinase leads to loss of Mcm4,6,7 helicase activity.
Ishimi Y, Komamura-Kohno Y. Ishimi Y, et al. J Biol Chem. 2001 Sep 14;276(37):34428-33. doi: 10.1074/jbc.M104480200. Epub 2001 Jul 13. J Biol Chem. 2001. PMID: 11454864 - The role of cyclin E in the regulation of entry into S phase.
Sauer K, Lehner CF. Sauer K, et al. Prog Cell Cycle Res. 1995;1:125-39. doi: 10.1007/978-1-4615-1809-9_10. Prog Cell Cycle Res. 1995. PMID: 9552358 Review. - [G2 phase cell cycle control and checkpoint].
Nagata A. Nagata A. Tanpakushitsu Kakusan Koso. 1996 Sep;41(12 Suppl):1833-8. Tanpakushitsu Kakusan Koso. 1996. PMID: 8890644 Review. Japanese. No abstract available.
Cited by
- Distinct activities of the anaphase-promoting complex/cyclosome (APC/C) in mouse embryonic cells.
Yang VS, Carter SA, Ng Y, Hyland SJ, Tachibana-Konwalski K, Fisher RA, Sebire NJ, Seckl MJ, Pedersen RA, Laskey RA, Gonzalez MA. Yang VS, et al. Cell Cycle. 2012 Mar 1;11(5):846-55. doi: 10.4161/cc.11.5.19251. Epub 2012 Mar 1. Cell Cycle. 2012. PMID: 22333576 Free PMC article. - Specialized roles of the two mitotic cyclins in somatic cells: cyclin A as an activator of M phase-promoting factor.
Fung TK, Ma HT, Poon RY. Fung TK, et al. Mol Biol Cell. 2007 May;18(5):1861-73. doi: 10.1091/mbc.e06-12-1092. Epub 2007 Mar 7. Mol Biol Cell. 2007. PMID: 17344473 Free PMC article. - Cyclin A/cdk2 regulates adenomatous polyposis coli-dependent mitotic spindle anchoring.
Beamish H, de Boer L, Giles N, Stevens F, Oakes V, Gabrielli B. Beamish H, et al. J Biol Chem. 2009 Oct 16;284(42):29015-23. doi: 10.1074/jbc.M109.042820. Epub 2009 Aug 24. J Biol Chem. 2009. PMID: 19703905 Free PMC article. - Histone deacetylation of RB-responsive promoters: requisite for specific gene repression but dispensable for cell cycle inhibition.
Siddiqui H, Solomon DA, Gunawardena RW, Wang Y, Knudsen ES. Siddiqui H, et al. Mol Cell Biol. 2003 Nov;23(21):7719-31. doi: 10.1128/MCB.23.21.7719-7731.2003. Mol Cell Biol. 2003. PMID: 14560017 Free PMC article. - Transactivation of murine cyclin A by polyomavirus large and small T antigens.
Schüchner S, Nemethova M, Belisova A, Klucky B, Holnthoner W, Wintersberger E. Schüchner S, et al. J Virol. 2001 Jul;75(14):6498-507. doi: 10.1128/JVI.75.14.6498-6507.2001. J Virol. 2001. PMID: 11413317 Free PMC article.
References
- Bates S., Ryan K.M., Phillips A.C., Vousden K.H. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene. 1998;17:1691–1703. - PubMed
- Boddy M.N., Furnari B., Mondesert O., Russell P. Replication checkpoint enforced by kinases Cds1 and Chk1. Science. 1998;280:909–912. - PubMed
- Bunz F., Dutriaux A., Lengauer C., Waldman T., Zhou S., Brown J.P., Sedivy J.M., Kinzler K.W., Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. - PubMed
- Cardoso M.C., Leonhardt H., Nadal-Ginard B. Reversal of terminal differentiation and control of DNA replicationcyclin A and Cdk2 specifically localize at subnuclear sites of DNA replication. Cell. 1993;74:979–992. - PubMed