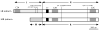UNC-13 is required for synaptic vesicle fusion in C. elegans - PubMed (original) (raw)
UNC-13 is required for synaptic vesicle fusion in C. elegans
J E Richmond et al. Nat Neurosci. 1999 Nov.
Abstract
We analyzed the synaptic physiology of unc-13 mutants in the nematode C. elegans. Mutants of unc-13 had normal nervous system architecture, and the densities of synapses and postsynaptic receptors were normal at the neuromuscular junction. However, the number of synaptic vesicles at neuromuscular junctions was two- to threefold greater in unc-13 mutants than in wild-type animals. Most importantly, evoked release at both GABAergic and cholinergic synapses was almost absent in unc-13 null alleles, as determined by whole-cell, voltage-clamp techniques. Although mutant synapses had morphologically docked vesicles, these vesicles were not competent for release as assayed by spontaneous release in calcium-free solution or by the application of hyperosmotic saline. These experiments support models in which UNC-13 mediates either fusion of vesicles during exocytosis or priming of vesicles for fusion.
Figures
Fig 1
Domain structure of UNC-13, indicating the location of the mutations used in this study. The e1091 and e51 mutations are stop codons in the L region and thus represent null alleles of the LR isoform. The s69 mutation is a five-basepair deletion in the R region and causes a frameshift in the common domain of the protein isoforms (Kohn and Rand, personal communication). The region between C2B and C2C is highly conserved among C. elegans, Drosophila and mammalian UNC-13 proteins; because s69 eliminates this region, this allele is likely to be a null mutation in both the LR and MR isoforms. The n2813 mutation is a missense change in the common R domain and thus represents a hypomorphic allele that affects all isoforms.
Fig 2
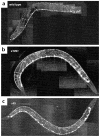
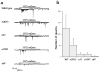
GABAergic motor neuron anatomy is normal in unc-13 mutants. The oxIs12 integrated array was used to label GABAergic neurons in the wild type (a), unc-13(e1091) (b) and unc-13(s69) (c). The oxIs12 array includes a construct with green fluorescent protein (GFP) expressed under the control of the unc-47 promoter, which is expressed in all GABAergic neurons. For all figures, anterior is to the right, dorsal is up.
Fig. 3
The density and distribution of neuromuscular synapses is normal in unc-13 mutants. (a–c) GFP-tagged synaptobrevin (SNB-GFP) localization. The integrated array nIs52 was used to label GABAergic neuromuscular junctions in the wild type (a), unc-13(e1091) (b) and unc-13(s69) (c). The nIs52 array includes a construct with GFP fused to the lumenal terminus of the synaptic vesicle protein synaptobrevin, under the regulation of the unc-25 promoter, which is expressed in GABAergic neurons. (d–f) Localization of postsynaptic GABA receptors (GABAR-GFP). The integrated array oxIs22 was used to visualize GABA receptors in the wild type (d), unc-13(e1091) (e) and unc-13(s69) (f). The oxIs22 array contains the unc-49 locus with GFP inserted into the intracellular loop of the UNC-49B GABA receptor subunit. (g) Interval between synapses (μm), measured as distance between SNB-GFP clusters. (h) Sample currents evoked by pressure ejection of GABA or acetylcholine (0.1 mM, 100 ms) onto body wall muscles of wild-type animals or unc-13 mutants. (i) Average current amplitudes observed in response to GABA and acetylcholine application.
Fig. 4
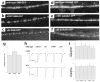
Synaptic vesicles accumulate in unc-13 mutants. Electron micrograph of GABAergic synapses in the wild type (a), unc-13(e1091) (b) and unc-13(s69) (c). In all micrographs, arrows indicate presynaptic specialization, and arrowheads indicate docked vesicles. (d) Average number of synaptic vesicles per active zone. These data include both GABAergic and cholinergic neuromuscular junctions. (e) Average number of docked vesicles per active zone. (f) Percent of vesicles docked at active zones. **p < 0.001, ***p < 0.0001 (significantly different from wild type) - difference from wild type not statistically significant.
Fig. 5
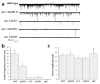
Evoked responses are reduced in unc-13 mutants. (a) Sample traces of evoked release in 5 mM Ca2+ and in 0 mM Ca2+ with 0.5 mM EGTA. (b) Sample traces of evoked responses in the muscles of the wild type, unc-13(n2813), unc-13(e51), unc-13(e1091) and unc-13(s69). (c) Average amplitudes of evoked currents in wild-type and unc-13 muscles.
Fig. 6
Endogenous release of synaptic vesicles is reduced in unc-13 mutants. (a) Sample traces of spontaneous currents in the muscles of the wild type, unc-13(n2813), unc-13(e51), unc-13(e1091) and unc-13(s69). (b) Average frequencies of spontaneous currents in wild-type and unc-13 muscles. (c) Average amplitudes of spontaneous events in wild-type and unc-13 muscles.
Fig. 7
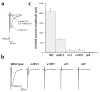
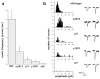
Calcium-free miniature postsynaptic events are severely reduced in unc-13 mutants. (a) Average frequency of calcium-free miniature postsynaptic currents in wild type (n = 7) and unc-13 muscles (n2813, n = 5; e51, n = 5; e1091, n = 5; s69, n = 3). (b) Left, amplitude histograms of calcium-free miniature currents in wild-type animals and unc-13 mutants. Right, sample traces.
Fig. 8
Release of synaptic vesicles is reduced in response to hyperosmotic media in unc-13 mutants. (a) Sample traces of postsynaptic currents before, during and after application of hyperosmotic medium to wild-type animals and unc-13 mutants. (b) Total quanta released by application of hyperosmotic media in wild-type animals (n = 7) and unc-13 mutants (n = 5).
Similar articles
- Caenorhabditis elegans rab-3 mutant synapses exhibit impaired function and are partially depleted of vesicles.
Nonet ML, Staunton JE, Kilgard MP, Fergestad T, Hartwieg E, Horvitz HR, Jorgensen EM, Meyer BJ. Nonet ML, et al. J Neurosci. 1997 Nov 1;17(21):8061-73. doi: 10.1523/JNEUROSCI.17-21-08061.1997. J Neurosci. 1997. PMID: 9334382 Free PMC article. - Tomosyn inhibits synaptic vesicle priming in Caenorhabditis elegans.
Gracheva EO, Burdina AO, Holgado AM, Berthelot-Grosjean M, Ackley BD, Hadwiger G, Nonet ML, Weimer RM, Richmond JE. Gracheva EO, et al. PLoS Biol. 2006 Jul;4(8):e261. doi: 10.1371/journal.pbio.0040261. PLoS Biol. 2006. PMID: 16895441 Free PMC article. - A post-docking role for active zone protein Rim.
Koushika SP, Richmond JE, Hadwiger G, Weimer RM, Jorgensen EM, Nonet ML. Koushika SP, et al. Nat Neurosci. 2001 Oct;4(10):997-1005. doi: 10.1038/nn732. Nat Neurosci. 2001. PMID: 11559854 Free PMC article. - The synaptic vesicle cycle: exocytosis and endocytosis in Drosophila and C. elegans.
Richmond JE, Broadie KS. Richmond JE, et al. Curr Opin Neurobiol. 2002 Oct;12(5):499-507. doi: 10.1016/s0959-4388(02)00360-4. Curr Opin Neurobiol. 2002. PMID: 12367628 Review. - Vesicular neurotransmitter transporters. Potential sites for the regulation of synaptic function.
Varoqui H, Erickson JD. Varoqui H, et al. Mol Neurobiol. 1997 Oct;15(2):165-91. doi: 10.1007/BF02740633. Mol Neurobiol. 1997. PMID: 9396009 Review.
Cited by
- Syntaxin opening by the MUN domain underlies the function of Munc13 in synaptic-vesicle priming.
Yang X, Wang S, Sheng Y, Zhang M, Zou W, Wu L, Kang L, Rizo J, Zhang R, Xu T, Ma C. Yang X, et al. Nat Struct Mol Biol. 2015 Jul;22(7):547-54. doi: 10.1038/nsmb.3038. Epub 2015 Jun 1. Nat Struct Mol Biol. 2015. PMID: 26030875 Free PMC article. - Molecular and circuit mechanisms underlying avoidance of rapid cooling stimuli in C. elegans.
Lin C, Shan Y, Wang Z, Peng H, Li R, Wang P, He J, Shen W, Wu Z, Guo M. Lin C, et al. Nat Commun. 2024 Jan 5;15(1):297. doi: 10.1038/s41467-023-44638-5. Nat Commun. 2024. PMID: 38182628 Free PMC article. - The role of laminins in the organization and function of neuromuscular junctions.
Rogers RS, Nishimune H. Rogers RS, et al. Matrix Biol. 2017 Jan;57-58:86-105. doi: 10.1016/j.matbio.2016.08.008. Epub 2016 Sep 7. Matrix Biol. 2017. PMID: 27614294 Free PMC article. Review. - Co-option of neurotransmitter signaling for inter-organismal communication in C. elegans.
Chute CD, DiLoreto EM, Zhang YK, Reilly DK, Rayes D, Coyle VL, Choi HJ, Alkema MJ, Schroeder FC, Srinivasan J. Chute CD, et al. Nat Commun. 2019 Jul 18;10(1):3186. doi: 10.1038/s41467-019-11240-7. Nat Commun. 2019. PMID: 31320626 Free PMC article. - A natural variant and engineered mutation in a GPCR promote DEET resistance in C. elegans.
Dennis EJ, Dobosiewicz M, Jin X, Duvall LB, Hartman PS, Bargmann CI, Vosshall LB. Dennis EJ, et al. Nature. 2018 Oct;562(7725):119-123. doi: 10.1038/s41586-018-0546-8. Epub 2018 Sep 26. Nature. 2018. PMID: 30258230 Free PMC article.
References
- Hosono R, Sassa T, Kuno S. Spontaneous mutations of trichlorfon resistance in the nematode, Caenorhabditis elegans. Zoological Sci. 1989;6:697–708.
Publication types
MeSH terms
Substances
Grants and funding
- R01 NS034307/NS/NINDS NIH HHS/United States
- R01 NS34307/NS/NINDS NIH HHS/United States
- R03 MH059820-01/MH/NIMH NIH HHS/United States
- R03 MHS9820-01/MH/NIMH NIH HHS/United States
- R03 MH059820-02/MH/NIMH NIH HHS/United States
- R03 HS009820/HS/AHRQ HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases