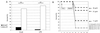Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide - PubMed (original) (raw)
Augmentation of innate host defense by expression of a cathelicidin antimicrobial peptide
R Bals et al. Infect Immun. 1999 Nov.
Abstract
Antimicrobial peptides, such as defensins or cathelicidins, are effector substances of the innate immune system and are thought to have antimicrobial properties that contribute to host defense. The evidence that vertebrate antimicrobial peptides contribute to innate immunity in vivo is based on their expression pattern and in vitro activity against microorganisms. The goal of this study was to investigate whether the overexpression of an antimicrobial peptide results in augmented protection against bacterial infection. C57BL/6 mice were given an adenovirus vector containing the cDNA for LL-37/hCAP-18, a human cathelicidin antimicrobial peptide. Mice treated with intratracheal LL-37/hCAP-18 vector had a lower bacterial load and a smaller inflammatory response than did untreated mice following pulmonary challenge with Pseudomonas aeruginosa PAO1. Systemic expression of LL-37/hCAP-18 after intravenous injection of recombinant adenovirus resulted in improved survival rates following intravenous injection of lipopolysaccharide with galactosamine or Escherichia coli CP9. In conclusion, the data demonstrate that expression of an antimicrobial peptide by gene transfer results in augmentation of the innate immune response, providing support for the hypothesis that vertebrate antimicrobial peptides protect against microorganisms in vivo.
Figures
FIG. 1
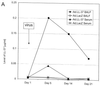
LL-37 in BALF and serum after administration of the recombinant virus or synthetic peptide. (A) Levels of LL-37 in BALF as determined by quantitative dot blot analysis. Virus was injected on day 1 of the experiment, and concentrations in BALF were determined on the following days. (B) Levels of LL-37 in serum as determined by quantitative dot blot analysis. Virus or peptide was injected on day 1 of the experiment, and concentrations in serum were determined by bleeding the animals and using the serum for qualitative dot blot analysis. (C) Western blots following denaturing polyacrylamide gel electrophoresis under reducing conditions with Tricine gels of mouse serum and BALF, using a polyclonal antibody against LL-37/hCAP-18. Lanes: 1, 20 ng of synthetic LL-37 peptide; 2, serum from a mouse that received the control vector coding for β-galactosidase; 3, serum from a mouse that received the vector coding for LL-37/hCAP-18 (crude); 4, serum from a mouse that received the vector coding for LL-37/hCAP-18 (RP-HPLC purified); 5, 20 ng of synthetic LL-37 peptide; 6, BALF from a mouse that received the vector coding for β-galactosidase (crude); 7 and 8, BALF from a mouse that received the vector coding for LL-37/hCAP-18 (crude, lane 7; HPLC purified, lane 8).
FIG. 1
LL-37 in BALF and serum after administration of the recombinant virus or synthetic peptide. (A) Levels of LL-37 in BALF as determined by quantitative dot blot analysis. Virus was injected on day 1 of the experiment, and concentrations in BALF were determined on the following days. (B) Levels of LL-37 in serum as determined by quantitative dot blot analysis. Virus or peptide was injected on day 1 of the experiment, and concentrations in serum were determined by bleeding the animals and using the serum for qualitative dot blot analysis. (C) Western blots following denaturing polyacrylamide gel electrophoresis under reducing conditions with Tricine gels of mouse serum and BALF, using a polyclonal antibody against LL-37/hCAP-18. Lanes: 1, 20 ng of synthetic LL-37 peptide; 2, serum from a mouse that received the control vector coding for β-galactosidase; 3, serum from a mouse that received the vector coding for LL-37/hCAP-18 (crude); 4, serum from a mouse that received the vector coding for LL-37/hCAP-18 (RP-HPLC purified); 5, 20 ng of synthetic LL-37 peptide; 6, BALF from a mouse that received the vector coding for β-galactosidase (crude); 7 and 8, BALF from a mouse that received the vector coding for LL-37/hCAP-18 (crude, lane 7; HPLC purified, lane 8).
FIG. 1
LL-37 in BALF and serum after administration of the recombinant virus or synthetic peptide. (A) Levels of LL-37 in BALF as determined by quantitative dot blot analysis. Virus was injected on day 1 of the experiment, and concentrations in BALF were determined on the following days. (B) Levels of LL-37 in serum as determined by quantitative dot blot analysis. Virus or peptide was injected on day 1 of the experiment, and concentrations in serum were determined by bleeding the animals and using the serum for qualitative dot blot analysis. (C) Western blots following denaturing polyacrylamide gel electrophoresis under reducing conditions with Tricine gels of mouse serum and BALF, using a polyclonal antibody against LL-37/hCAP-18. Lanes: 1, 20 ng of synthetic LL-37 peptide; 2, serum from a mouse that received the control vector coding for β-galactosidase; 3, serum from a mouse that received the vector coding for LL-37/hCAP-18 (crude); 4, serum from a mouse that received the vector coding for LL-37/hCAP-18 (RP-HPLC purified); 5, 20 ng of synthetic LL-37 peptide; 6, BALF from a mouse that received the vector coding for β-galactosidase (crude); 7 and 8, BALF from a mouse that received the vector coding for LL-37/hCAP-18 (crude, lane 7; HPLC purified, lane 8).
FIG. 2
Expression of LL-37/hCAP-18 in mouse liver and lungs after gene transfer. Organs were harvested 5 days after gene transfer and analyzed for transgene expression by RT-PCR and immunohistochemistry. Immunohistochemistry with polyclonal antibodies to LL-37/hCAP-18 revealed signals in hepatocytes (B) or epithelial cells of airways (D) of mice that received LL-37 vector but not in those of mice treated with Ad.AlkPhos vector (A and C). Bar, 100 μm. (E) RT-PCR was performed with LL-37/hCAP-18-specific primers and revealed the presence of transcripts only in mice after application of LL-37 vector. Amplification of glyceraldehyde-3-phosphate dehydrogenase (G3PDH) was used as a positive control. The PCR products were blotted and hybridized to an LL-37/hCAP-18-specific probe. Lane LL-37, positive control with plasmid DNA; lanes 1 and 2, PCR on RNA extracted from lungs (lane 1) or liver (lane 2) obtained from a mouse treated with lacZ vector; lanes 3 and 4, PCR on RNA extracted from lungs (lane 3) or liver (lane 4) obtained from a mouse treated with LL-37 vector.
FIG. 3
Effect of gene transfer of LL-37/hCAP-18 to the respiratory tracts of mice on the bacterial load and inflammatory response. Mice were injected intratracheally on day 1, challenged with bacteria on day 5, and euthanized on day 6. Individual data points are presented. The bar represents the mean. (A) The bacterial load was significantly decreased in mice that received the LL-37/hCAP-18-encoding vector (Ad.LL-37) (P < 0.005). (B) Levels of TNF-α were significantly lower in mice that received the LL-37/hCAP-18-encoding vector (P < 0.05) (40 mice per group).
FIG. 4
Effect of the systemic overexpression of LL-37/hCAP-18 in mice on survival after intraperitoneal injection of LPS (in galactosamine-sensitized mice) or gram-negative bacteria. (A) Mice received either lacZ or LL-37 vector on day 1 and were injected with LPS plus galactosamine or E. coli CP9 on day 5. Survival of the animals that were treated with LL-37 vector (Ad.LL-37) (5 × 1010 particles) was significantly (∗∗, P < 0.05) increased compared to survival of the mice that received the same dose of lacZ control vector (Ad.lacZ) (20 mice in each group). (B) Dose-dependent survival of animals treated with different amounts of LL-37/hCAP-18-encoding virus. Whereas the control group treated with β-galactosidase-encoding vector showed high mortality after injection, the animals that received LL-37 vector showed increased survival rates that correlated with the amount of virus applied and therefore with the level of the LL-37 peptide in serum (10 mice in each group).
Similar articles
- Transient cutaneous adenoviral gene therapy with human host defense peptide hCAP-18/LL-37 is effective for the treatment of burn wound infections.
Jacobsen F, Mittler D, Hirsch T, Gerhards A, Lehnhardt M, Voss B, Steinau HU, Steinstraesser L. Jacobsen F, et al. Gene Ther. 2005 Oct;12(20):1494-502. doi: 10.1038/sj.gt.3302568. Gene Ther. 2005. PMID: 15973442 - The peptide antibiotic LL-37/hCAP-18 is expressed in epithelia of the human lung where it has broad antimicrobial activity at the airway surface.
Bals R, Wang X, Zasloff M, Wilson JM. Bals R, et al. Proc Natl Acad Sci U S A. 1998 Aug 4;95(16):9541-6. doi: 10.1073/pnas.95.16.9541. Proc Natl Acad Sci U S A. 1998. PMID: 9689116 Free PMC article. - Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37.
Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA. Krasnodembskaya A, et al. Stem Cells. 2010 Dec;28(12):2229-38. doi: 10.1002/stem.544. Stem Cells. 2010. PMID: 20945332 Free PMC article. - Pulmonary defense and the human cathelicidin hCAP-18/LL-37.
Fahy RJ, Wewers MD. Fahy RJ, et al. Immunol Res. 2005;31(2):75-89. doi: 10.1385/IR:31:2:075. Immunol Res. 2005. PMID: 15778507 Free PMC article. Review. - Cutaneous defense mechanisms by antimicrobial peptides.
Braff MH, Bardan A, Nizet V, Gallo RL. Braff MH, et al. J Invest Dermatol. 2005 Jul;125(1):9-13. doi: 10.1111/j.0022-202X.2004.23587.x. J Invest Dermatol. 2005. PMID: 15982297 Review.
Cited by
- A model for antimicrobial gene therapy: demonstration of human beta-defensin 2 antimicrobial activities in vivo.
Huang GT, Zhang HB, Kim D, Liu L, Ganz T. Huang GT, et al. Hum Gene Ther. 2002 Nov 20;13(17):2017-25. doi: 10.1089/10430340260395875. Hum Gene Ther. 2002. PMID: 12489997 Free PMC article. - Protecting the boundary: the sentinel role of host defense peptides in the skin.
Bernard JJ, Gallo RL. Bernard JJ, et al. Cell Mol Life Sci. 2011 Jul;68(13):2189-99. doi: 10.1007/s00018-011-0712-8. Epub 2011 May 15. Cell Mol Life Sci. 2011. PMID: 21573782 Free PMC article. Review. - Antimicrobial Activity of Mesenchymal Stem Cells: Current Status and New Perspectives of Antimicrobial Peptide-Based Therapies.
Alcayaga-Miranda F, Cuenca J, Khoury M. Alcayaga-Miranda F, et al. Front Immunol. 2017 Mar 30;8:339. doi: 10.3389/fimmu.2017.00339. eCollection 2017. Front Immunol. 2017. PMID: 28424688 Free PMC article. Review. - Evaluation of circulating serum cathelicidin levels as a potential biomarker to discriminate between active and latent tuberculosis in Uganda.
Acen EL, Kateete DP, Worodria W, Olum R, Joloba ML, Bbuye M, Biraro IA. Acen EL, et al. PLoS One. 2022 Aug 26;17(8):e0272788. doi: 10.1371/journal.pone.0272788. eCollection 2022. PLoS One. 2022. PMID: 36018845 Free PMC article. - The Utility of Noninvasive Urinary Biomarkers for the Evaluation of Vesicoureteral Reflux in Children.
Colceriu MC, Aldea PL, Boț Răchişan AL, Bulată B, Delean D, Grama A, Mititelu A, Decea RM, Sevastre-Berghian A, Clichici S, Pop TL, Mocan T. Colceriu MC, et al. Int J Mol Sci. 2023 Dec 17;24(24):17579. doi: 10.3390/ijms242417579. Int J Mol Sci. 2023. PMID: 38139407 Free PMC article.
References
- Bensch K W, Raida M, Magert H-J, Schulz-Knappe P, Forssmann W-G. hBD-1: a novel β-defensin from human plasma. FEBS Lett. 1995;368:331–335. - PubMed
- Engelhardt J F, Yang Y, Stratford-Perricaudet L D, Allen E D, Kozarsky K, Perricaudet M, Yankaskas J R, Wilson J M. Direct gene transfer of human CFTR into human bronchial epithelia of xenografts with E1-deleted adenoviruses. Nat Genet. 1993;4:27–34. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL 49040/HL/NHLBI NIH HHS/United States
- P50 DK049136/DK/NIDDK NIH HHS/United States
- P50 DK49136/DK/NIDDK NIH HHS/United States
- P30 DK047757/DK/NIDDK NIH HHS/United States
- P30 DK47757/DK/NIDDK NIH HHS/United States
- R01 HL049040/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical