Excitatory synaptogenesis between identified Lymnaea neurons requires extrinsic trophic factors and is mediated by receptor tyrosine kinases - PubMed (original) (raw)
Excitatory synaptogenesis between identified Lymnaea neurons requires extrinsic trophic factors and is mediated by receptor tyrosine kinases
T Hamakawa et al. J Neurosci. 1999.
Abstract
Neurotrophic factors have well established roles in neuronal development and adult synaptic plasticity, but their precise role in synapse formation has yet to be determined. This paper provides the first direct evidence that neurotrophic factors in brain conditioned medium (CM) differentially regulate excitatory and inhibitory synapse formation. Somata of identified presynaptic and postsynaptic neurons were isolated from the CNS of Lymnaea and were cultured in a soma-soma configuration in the presence (CM) or absence [defined medium (DM)] of trophic factors. In DM, excitatory synapses did not form. When they were paired in CM or in DM containing Lymnaea epidermal growth factor (EGF); however, all presynaptic neurons reestablished their specific excitatory synapses, which had electrical properties similar to those seen in vivo. CM-induced formation of excitatory synapses required transcription and de novo protein synthesis, as indicated by the observations that synapse formation was blocked by the protein synthesis inhibitor anisomycin and the protein transcription blocker actinomycin D; the CM factor was inactivated by boiling. They were also blocked by receptor tyrosine kinase inhibitors (lavendustin A, genistein, K252a, and KT5926) but not by inactive analogs (genistin and lavendustin B), suggesting that the effect was mediated by receptor tyrosine kinases. These results, together with our previously published data, demonstrate that trophic factors are required for excitatory, but not inhibitory, synapse formation and extends the role of EGF from cell proliferation, neurite outgrowth, and survival to excitatory synapse formation.
Figures
Fig. 1.
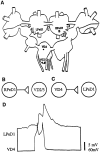
Diagrams indicating the position, location, and nature of synaptic connections between identified_Lymnaea_ neurons used in the present study.A, Ganglionic location of identified neurons right pedal dorsal 1 (RPeD1), left pedal dorsal 1 (LPeD1), visceral dorsal 4 (VD4), and visceral dorsal 2/3 (VD2/3), which are located in the right pedal, left pedal, and visceral ganglia, respectively. Note that VD2 and 3 are thought to be electrophysiologically and morphologically identical and are therefore simply referred to as VD2/3 (Magoski and Bulloch, 1997). B, Diagrammatic representation of the in vivo excitatory synaptic connection between RPeD1 and VD2/3 (Winlow and Benjamin, 1977; Magoski et al., 1995; Magoski and Bulloch, 1997). RPeD1 forms an excitatory synapse with VD2/3. C, Diagrammatic representation of the in vivo synaptic connection between VD4 and LPeD1. VD4 forms an excitatory synapse with LPeD1. D, Simultaneous intracellular recording in an isolated ganglionic preparation revealed an excitatory synapse between VD4 and LPeD1; action potentials in VD4 produced 1:1 EPSPs in LPeD1.
Fig. 2.
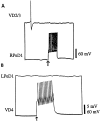
Excitatory synapses between soma–soma paired neurons fail to develop in DM. After 18–24 hr of cell pairing in DM, simultaneous intracellular recordings revealed that a burst of action potentials in RPeD1 failed to generate a postsynaptic potential in VD2/3 (A). Likewise, a burst of action potentials in VD4 failed to alter the membrane potential of LPeD1 (B).
Fig. 3.
Appropriate excitatory synapses are reestablished between soma–soma paired neurons in CM. After 18–24 hr of soma–soma pairing in CM, simultaneous intracellular recordings showed that action potentials in RPeD1 produced 1:1 EPSPs in VD2/3 (A). B, Similarly, action potentials in VD4 produced 1:1 EPSPs in LPeD1.
Fig. 4.
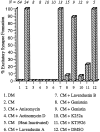
CM-induced synaptogenesis between RPeD1 and VD2/3 requires transcription, de novo protein synthesis, and receptor tyrosine kinases. In DM, an appropriate excitatory synapse fails to develop between RPeD1 and VD2/3. When paired in CM, however, appropriate excitatory synapses reestablish between soma–soma paired RPeD1 and VD2/3. CM-induced excitatory synapse formation is blocked, however, when paired cells are incubated in CM containing either anisomycin (protein synthesis inhibitor) or actinomycin D (protein transcription inhibitor). Heat-inactivated CM (boiled at 100°C for 20 min) also fails to support excitatory synapse formation. CM-induced excitatory synapse formation was partially or completely blocked by the addition of receptor tyrosine kinase inhibitors (lavendustin A, genistein, K252a, or KT5926) but not by inactive forms of receptor tyrosine kinase inhibitors (lavendustin B and genistin). Similarly, addition of the carrier solution (DMSO) to CM did not perturb synapse formation between RPeD1 and VD2/3. N values for each experiment are indicated above the data bars.
Fig. 5.
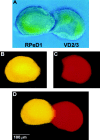
CM-induced excitatory synaptogenesis does not involve neurite outgrowth from soma–soma paired cells. To test whether CM-induced excitatory synapse formation between soma–soma paired cells (A) involved neurite outgrowth, both presynaptic (RPeD1) and postsynaptic (VD2/3) neurons were injected with fluorescent markers. Specifically, neurons were injected iontophoretically with either Lucifer yellow (RPeD1; B) or sulforhodamine (VD2/3; C) and viewed by fluorescence microscopy. Neither RPeD1 (B, D) nor VD2/3 (C, D) exhibited neurite outgrowth in CM.
Fig. 6.
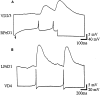
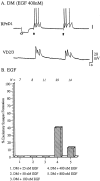
CM-induced effects on excitatory synaptogenesis are mimicked by Lymnaea EGF. Identified neurons RPeD1 and VD2/3 were soma–soma paired in DM containing various concentrations of Lymnaea EGF. A, EGF (400 n
m
) induced synaptogenesis between soma–soma paired RPeD1 and VD2/3. Specifically, action potentials in RPeD1 (solid arrow) produced 1:1 EPSPs in VD2/3. The injection of a hyperpolarizing current in RPeD1 (open arrow) did not reveal electrical coupling between the cells. B, The relationship between EGF concentration and excitatory synaptogenesis between RPeD1 and VD2/3 was most similar to an inverted U-shaped function.
Similar articles
- Trophic factor-induced excitatory synaptogenesis involves postsynaptic modulation of nicotinic acetylcholine receptors.
Woodin MA, Munno DW, Syed NI. Woodin MA, et al. J Neurosci. 2002 Jan 15;22(2):505-14. doi: 10.1523/JNEUROSCI.22-02-00505.2002. J Neurosci. 2002. PMID: 11784796 Free PMC article. - Different extrinsic trophic factors regulate neurite outgrowth and synapse formation between identified Lymnaea neurons.
Munno DW, Woodin MA, Lukowiak K, Syed NI, Dickinson PS. Munno DW, et al. J Neurobiol. 2000 Jul;44(1):20-30. doi: 10.1002/1097-4695(200007)44:1<20::aid-neu3>3.0.co;2-m. J Neurobiol. 2000. PMID: 10880129 - Trophic factor-induced plasticity of synaptic connections between identified Lymnaea neurons.
Woodin MA, Hamakawa T, Takasaki M, Lukowiak K, Syed NI. Woodin MA, et al. Learn Mem. 1999 May-Jun;6(3):307-16. Learn Mem. 1999. PMID: 10492012 Free PMC article. - [Electrical nature of the biphasic (excitatory-inhibitory) postsynaptic potential transmitted between 2 giant neurons from Aplysia].
Meunier JM, Tauc L. Meunier JM, et al. Arch Ital Biol. 1973 Dec;111(3-4):305-22. Arch Ital Biol. 1973. PMID: 18847033 Review. French. No abstract available. - Molecular diversity underlying cortical excitatory and inhibitory synapse development.
Favuzzi E, Rico B. Favuzzi E, et al. Curr Opin Neurobiol. 2018 Dec;53:8-15. doi: 10.1016/j.conb.2018.03.011. Epub 2018 Apr 25. Curr Opin Neurobiol. 2018. PMID: 29704699 Review.
Cited by
- In vitro studies of neuronal networks and synaptic plasticity in invertebrates and in mammals using multielectrode arrays.
Massobrio P, Tessadori J, Chiappalone M, Ghirardi M. Massobrio P, et al. Neural Plast. 2015;2015:196195. doi: 10.1155/2015/196195. Epub 2015 Mar 17. Neural Plast. 2015. PMID: 25866681 Free PMC article. Review. - Neurotrophic actions of a novel molluscan epidermal growth factor.
Hermann PM, van Kesteren RE, Wildering WC, Painter SD, Reno JM, Smith JS, Kumar SB, Geraerts WP, Ericsson LH, Smit AB, Bulloch AG, Nagle GT. Hermann PM, et al. J Neurosci. 2000 Sep 1;20(17):6355-64. doi: 10.1523/JNEUROSCI.20-17-06355.2000. J Neurosci. 2000. PMID: 10964941 Free PMC article. - Reconsolidation of a long-term memory in Lymnaea requires new protein and RNA synthesis and the soma of right pedal dorsal 1.
Sangha S, Scheibenstock A, Lukowiak K. Sangha S, et al. J Neurosci. 2003 Sep 3;23(22):8034-40. doi: 10.1523/JNEUROSCI.23-22-08034.2003. J Neurosci. 2003. PMID: 12954865 Free PMC article. - The role of synaptotagmin I C2A calcium-binding domain in synaptic vesicle clustering during synapse formation.
Gardzinski P, Lee DW, Fei GH, Hui K, Huang GJ, Sun HS, Feng ZP. Gardzinski P, et al. J Physiol. 2007 May 15;581(Pt 1):75-90. doi: 10.1113/jphysiol.2006.127472. Epub 2007 Feb 22. J Physiol. 2007. PMID: 17317745 Free PMC article. - Activation of epidermal growth factor receptor mediates receptor axon sorting and extension in the developing olfactory system of the moth Manduca sexta.
Gibson NJ, Tolbert LP. Gibson NJ, et al. J Comp Neurol. 2006 Apr 10;495(5):554-72. doi: 10.1002/cne.20890. J Comp Neurol. 2006. PMID: 16498681 Free PMC article.
References
- Berninger B, Poo M-M. Fast actions of neurotrophic factors. Curr Opin Neurobiol. 1996;6:324–330. - PubMed
- Bjorgum MC, Hamakawa T, Takasaki M, Syed NI. Brain-conditioned medium induced excitatory synaptogenesis between identified Lymnaea neurons may require activity dependent mechanisms. Soc Neurosci Abstr. 1998;24:791.
- Boulanger L, Poo M-M. Presynaptic depolarization facilitates neurotrophin-induced synaptic potentiation. Nat Neurosci. 1999;2:345–351. - PubMed
- Cohen-Corey S, Fraser SE. Effects of brain-derived neurotrophic factor on optic axon branching and remodeling in vivo. Nature. 1995;378:192–196. - PubMed
- Fainzilber M, Smit AB, Syed NI, Wildering WC, Hermann PM, van der Schors RC, Jiménez C, Li KW, van Minnen J, Bulloch AGM, Ibáñez CF, Geraerts WPM. CRNF, a molluscan neurotrophic factor that interacts with the p75 neurotrophin receptor. Science. 1996;274:1540–1543. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources