Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice - PubMed (original) (raw)
Targeted deletion of a cyclic nucleotide-gated channel subunit (OCNC1): biochemical and morphological consequences in adult mice
H Baker et al. J Neurosci. 1999.
Abstract
The olfactory cyclic nucleotide-gated channel subunit 1 (OCNC1) is required for signal transduction in olfactory receptor cells. To further investigate the role of this channel in the olfactory system, the biochemical and morphological consequences of targeted disruption of OCNC1 were investigated in adult mice. Null as compared to wild-type mice had smaller olfactory bulbs, suggesting compromised development of the central target of the receptor cells. Ectopic olfactory marker protein (OMP)-stained fibers localized to the external plexiform layer reflected the relative immaturity of the olfactory bulb in the null mice. The olfactory epithelium of the knock-out mouse was thinner and showed lower expression of olfactory marker protein and growth-associated protein 43, indicating decreases in both generation and maturation of receptor cells. Tyrosine hydroxylase (TH) expression in the olfactory bulb, examined as a reflection of afferent activity, was reduced in the majority of periglomerular neurons but retained in atypical or "necklace" glomeruli localized to posterior aspects of the olfactory bulb. Double label studies demonstrated that the remaining TH-immunostained neurons received their innervation from a subset of receptor cells previously shown to express a phosphodiesterase that differs from that found in most receptor cells. These data indicate that expression of OCNC1 is required for normal development of the olfactory epithelium and olfactory bulb. The robust expression of TH in some periglomerular cells in the OCNC1-null mice suggests that receptor cells innervating these glomeruli may use an alternate signal transduction pathway.
Figures
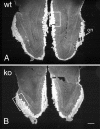
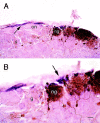
Fig. 1.
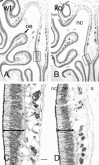
OMP immunostaining in horizontal sections of olfactory bulbs from adult wild-type (A,wt) and OCNC1-null (B, ko) mice illustrated in dark-field photomicrographs. Note the smaller size of the olfactory bulbs in the null mice, but the apparently normal OMP staining of the olfactory nerve (on) and glomerular (gl) layers. Boxes indicate regions shown at higher magnification in Figure 2. Scale bar, 200 μm.
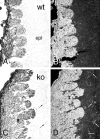
Fig. 2.
Bright-field (A, C) and dark-field (B, D) photomicrographs illustrating the presence of ectopic OMP-immunolabeled fibers in OCNC1-null (C, D; ko) and not in wild-type (A, B, wt) mouse olfactory bulbs. Fibers (arrows) are scattered throughout the external plexiform layer (epl) of the null mice. Scale bar, 40 μm.
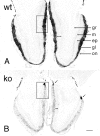
Fig. 3.
Bright-field photomicrographs of TH immunostaining in wild-type (A, wt) and OCNC1-null (B, ko) mice. In wild-type mice, TH staining occurs in periglomerular cells surrounding all glomeruli and in fibers within the glomeruli. In the null mice, the majority of glomeruli have only a few TH-labeled neurons and display a dramatic reduction in fiber staining within the glomeruli. However, normal TH staining is found in a group of glomeruli (arrows) on posterior aspects of the olfactory bulb. _Double arrows_indicate the decrease in width of the external plexiform layer in OCNC1-null mice. ep, External plexiform layer;gl, glomerular layer; gr, granule cell layer; m, mitral cell layer; on, olfactory nerve layer. Boxes indicate regions shown at higher magnification in Figure 4. Scale bar, 200 μm.
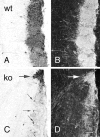
Fig. 4.
Bright-field (A, C) and dark-field (B, D) photomicrographs of TH immunostaining of olfactory bulb in wild-type (A, B; wt) and OCNC1-null (C, D; ko) mice. In wild-type mice, strong staining is observed in periglomerular cells and glomerular processes of all glomeruli. In the null mice, only scattered periglomerular cells (small arrows) contain TH in most glomeruli and, as illustrated in dark-field (D), labeled processes are absent. In contrast, TH staining is normal in both cells and processes in the atypical or necklace glomeruli (large arrow). Scale bar, 40 μm.
Fig. 5.
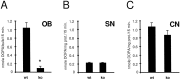
TH activity in the olfactory bulb (OB), substantia nigra (SN), and caudate nucleus (CN) of wild-type (wt) and OCNC1-null (ko) mice. In the null mice, TH activity is normal in SN and CN, but lower only in the olfactory bulb. Student's unpaired t test; *p < 0.01; n = 3–10.
Fig. 6.
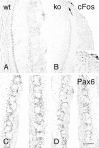
Bright-field photomicrographs illustrating cFos (A, B) and Pax6 (C, D) immunostaining in the olfactory bulbs of wild-type (A, C; wt) and OCNC1-null (B, D; ko) mice. In null mice, cFos is absent in most glomeruli but staining is present in the region of the necklace glomeruli (arrow, inset). Pax6 immunostaining is present in the olfactory bulbs of both wt and ko mice. Scale bar: A–D, 120 μm;inset, 60 μm.
Fig. 7.
OMP immunostaining in the olfactory epithelium of wild-type (A, C; wt) and OCNC1-null (B, D; ko) mice. Low-power micrographs (A, B) illustrate that the receptor epithelium (oe) is thinner (C, _D,compare length of double arrows) in all regions of the nasal cavity (nc). The boxed areas in_A and B are shown at higher magnification in C and D. lp, Lamina propria; s, nasal septum. Scale bar: A,B, 250 μm; C, D, 30 μm.
Fig. 8.
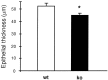
Bar graph illustrating that the septal receptor epithelium is 15% thinner in OCNC1-null mice (ko) as compared to wild-type mice (wt). n = 3 per group. *p < 0.05 by paired Student's_t_ test.
Fig. 9.
GAP43 immunostaining in the olfactory epithelium of wild-type (A, wt) and OCNC1-null (B, ko) mice. Fewer neurons (small arrows) are stained with GAP43, indicating that the number of both mature and immature neurons is lower in the null mice. The double arrows indicate the difference in the thickness of the olfactory epithelium (oe). lp, Lamina propria; nc, nasal cavity; s, septum. Scale bar, 30 μm.
Fig. 10.
Low-power (A) and high-power (B) photomicrographs illustrating that only the necklace glomeruli (ng) that display intense TH immunoreactivity are innervated by PDE fibers. Most glomeruli (g) show no tyrosine hydroxylase immunoreactivity (brown) and are not innervated by PDE2-immunoreactive fibers (purple). The PDE2 fibers (A, arrow) course along the edge of the olfactory nerve layer (on) and innervate only the necklace glomeruli (B, arrow). Scale bar: A, 40 μm;B, 20 μm.
Similar articles
- Peripheral olfactory projections are differentially affected in mice deficient in a cyclic nucleotide-gated channel subunit.
Zheng C, Feinstein P, Bozza T, Rodriguez I, Mombaerts P. Zheng C, et al. Neuron. 2000 Apr;26(1):81-91. doi: 10.1016/s0896-6273(00)81140-x. Neuron. 2000. PMID: 10798394 - X inactivation of the OCNC1 channel gene reveals a role for activity-dependent competition in the olfactory system.
Zhao H, Reed RR. Zhao H, et al. Cell. 2001 Mar 9;104(5):651-60. doi: 10.1016/s0092-8674(01)00262-8. Cell. 2001. PMID: 11257220 - Central role of the CNGA4 channel subunit in Ca2+-calmodulin-dependent odor adaptation.
Munger SD, Lane AP, Zhong H, Leinders-Zufall T, Yau KW, Zufall F, Reed RR. Munger SD, et al. Science. 2001 Dec 7;294(5549):2172-5. doi: 10.1126/science.1063224. Science. 2001. PMID: 11739959 Free PMC article. - Odors detected by mice deficient in cyclic nucleotide-gated channel subunit A2 stimulate the main olfactory system.
Lin W, Arellano J, Slotnick B, Restrepo D. Lin W, et al. J Neurosci. 2004 Apr 7;24(14):3703-10. doi: 10.1523/JNEUROSCI.0188-04.2004. J Neurosci. 2004. PMID: 15071119 Free PMC article. - Olfactory epithelium progenitors: insights from transgenic mice and in vitro biology.
Murdoch B, Roskams AJ. Murdoch B, et al. J Mol Histol. 2007 Dec;38(6):581-99. doi: 10.1007/s10735-007-9141-2. Epub 2007 Sep 13. J Mol Histol. 2007. PMID: 17851769 Review.
Cited by
- Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction.
Petreanu L, Alvarez-Buylla A. Petreanu L, et al. J Neurosci. 2002 Jul 15;22(14):6106-13. doi: 10.1523/JNEUROSCI.22-14-06106.2002. J Neurosci. 2002. PMID: 12122071 Free PMC article. - Olfactory signal transduction in the mouse septal organ.
Ma M, Grosmaitre X, Iwema CL, Baker H, Greer CA, Shepherd GM. Ma M, et al. J Neurosci. 2003 Jan 1;23(1):317-24. doi: 10.1523/JNEUROSCI.23-01-00317.2003. J Neurosci. 2003. PMID: 12514230 Free PMC article. - Grueneberg ganglion olfactory subsystem employs a cGMP signaling pathway.
Liu CY, Fraser SE, Koos DS. Liu CY, et al. J Comp Neurol. 2009 Sep 1;516(1):36-48. doi: 10.1002/cne.22096. J Comp Neurol. 2009. PMID: 19565523 Free PMC article. - Sensory input, sex and function shape hypothalamic cell type development.
Kaplan HS, Logeman BL, Zhang K, Yawitz TA, Santiago C, Sohail N, Talay M, Seo C, Naumenko S, Ho Sui SJ, Ginty DD, Ren B, Dulac C. Kaplan HS, et al. Nature. 2025 Mar 5. doi: 10.1038/s41586-025-08603-0. Online ahead of print. Nature. 2025. PMID: 40044853 - Function and dysfunction of CNG channels: insights from channelopathies and mouse models.
Biel M, Michalakis S. Biel M, et al. Mol Neurobiol. 2007 Jun;35(3):266-77. doi: 10.1007/s12035-007-0025-y. Mol Neurobiol. 2007. PMID: 17917115 Review.
References
- Baker H. Unilateral, neonatal olfactory deprivation alters tyrosine hydroxylase expression but not aromatic amino acid decarboxylase or GABA immunoreactivity. Neuroscience. 1990;36:761–771. - PubMed
- Baker H, Farbman AI. Olfactory afferent regulation of the dopamine phenotype in the fetal rat olfactory system. Neuroscience. 1993;52:115–134. - PubMed
- Baker H, Kawano T, Albert VR, Joh TH, Reis DJ, Margolis FL. Olfactory bulb dopamine neurons survive deafferentiation induced loss of tyrosine hydroxylase. Neuroscience. 1984;11:605–615. - PubMed
- Baker H, Morel K, Stone DM, Maruniak JA. Adult naris closure profoundly reduces tyrosine hydroxylase expression in mouse olfactory bulb. Brain Res. 1993;614:109–116. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases