Regulation of learning by EphA receptors: a protein targeting study - PubMed (original) (raw)
Regulation of learning by EphA receptors: a protein targeting study
R Gerlai et al. J Neurosci. 1999.
Abstract
EphA family receptor tyrosine kinases and their ephrin-A ligands are involved in patterning axonal connections during brain development, but until now a role for these molecules in the mature brain had not been elucidated. Here, we show that both the EphA5 receptor and its ephrin-A ligands (2 and 5) are expressed in the adult mouse hippocampus, and the EphA5 protein is present in a phosphorylated form. Because there are no pharmacological agents available for EphA receptors, we designed recombinant immunoadhesins that specifically bind to the receptor binding site of the ephrin-A ligand (antagonist) or the ligand binding site of the EphA receptor (agonist) and thus target EphA function. We demonstrate that intrahippocampal infusion of an EphA antagonist immunoadhesin leads to impaired performance in two behavioral paradigms, T-maze spontaneous alternation and context-dependent fear conditioning, sensitive to hippocampal function, whereas activation of EphA by infusion of an agonist immunoadhesin results in enhanced performance on these tasks. Because the two behavioral tasks have different motivational, perceptual, and motor requirements, we infer the changes were not caused by these performance factors but rather to cognitive alterations. We also find bidirectional changes in gene expression and in electrophysiological measures of synaptic efficacy that correlate with the behavioral results. Thus, EphA receptors and their ligands are implicated as mediators of plasticity in the adult mammalian brain.
Figures
Fig. 1.
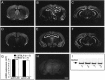
The EphA5 receptor and its ligands are expressed in the adult mouse hippocampus. In situ hybridization (coronal sections with antisense probe) for EphA5 mRNA expression in the brain of C57BL/6 (A–C) and DBA/2 (D–F) strains of mice. A section with sense (control) probe (H) is also shown. Approximate position of sections from bregma are (in mm):A, D, +0.75; B,E, H, −2.10; C,F, −3.00. Both C57BL6 and DBA/2 mice display prominent expression of EphA5 mRNA in hippocampus and dentate gyrus. Scale bar (in H), 1000 μm. CA, CA1–CA3 areas of the hippocampal formation; DG, dentate Gyrus;CX, cortex; TH, thalamus;HT, hippothalamus; AM, amygdala;PX, piriform cortex. G, Ephrin-A5 and -A2 ligands are expressed in the hippocampus of both inbred strains of mice analyzed. Real-time quantitative (TaqMan) RT-PCR for mRNA of ligands ephrin-A5 and -A2 in the hippocampi of C57BL/6 (black bars) and DBA/2 (white bars). The data (mean ± SEM) are based on the number of PCR amplification cycles required to reach a threshold level (cycles to threshold,CT) of cleavage of a fluorescent reporter probe (Gibson et al., 1996; Heid et al., 1996) and are normalized to GAPDH housekeeping gene transcript (Δ_CT_). Sample sizes (n) indicate the number of mice analyzed. Note that larger values mean smaller original mRNA amount in the hippocampal tissue sample. Also note that amplification characteristics are unique to each gene; therefore, comparison from one gene to another is not valid. I, Western blot for phosphorylated EphA5. Each lane represents a hippocampal tissue sample from an individual mouse. Both DBA/2 (a1, a2) and C57BL/6 (b1, b2) strain of mice exhibit a prominent signal.
Fig. 2.
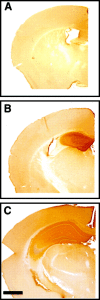
Immunohistochemical staining for the IgG domain of the immunoadhesin reveals pronounced hippocampal diffusion of the protein after 7 d intrahippocampal infusion from micro-osmotic pump. The right hemisphere with EphA5-IgG infusion in C57BL/6 mice is shown: A, anterior (bregma −0.70 mm);B, anterior (bregma −1.35 mm); C, posterior (bregma −2.50) to the cannula insertion site. Note the strong peroxidase (brown) staining observable in the hippocampus and the lack of staining in infrahippocampal areas. Note that staining of suprahippocampal cortical areas within 1 mm from the cannula insertion site was observed in some specimens. Scale bar, 1000 μm.
Fig. 3.
EphA5 receptor phosphorylation is induced by_in vivo_ infusion of the agonist ephrinA5-IgG in hippocampal tissue samples. After immunoprecipitation with anti-EphA5 antibody, phosphorylation levels were detected by anti-phosphotyrosine kinase antibody. Each lane represents hippocampal tissue from an individual mouse: a, ephrinA5-IgG infusion;b, CD4-IgG infusion. Lanes 1–4 represent samples from C57BL/6; lanes 5–8 represent samples from DBA/2. The monomer (EphA5) protein is indicated.
Fig. 4.
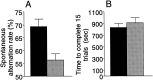
EphA5-IgG infusion impairs T-maze continuous alternation in C57BL/6 mice in a 15 trial alternation session. EphA5-IgG infusion, hatched bar, n = 23; CD4-IgG infusion, black bar, n = 22. One choice was allowed at each trial. Alternation rate is a ratio between the alternating choices and total number of choices.A, Significant difference was found in alternation rate (t = 3.528; df = 43; p < 0.001). _B_, No significant difference was found between groups in time spent to complete the 15 choices (_t_= 0.915; df = 43; _p_ > 0.36). Error bars represent SEM.
Fig. 5.
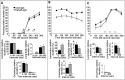
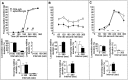
EphA5-IgG infusion impairs learning performance in a context-specific manner in fear conditioning in C57BL/6 mice. In the CDFC paradigm, mice associate two substantially different types of cues with a negative reinforcer, an electric foot shock. The shock is paired with a simple associative cue, a tone, in a shock chamber characterized by multiple contextual cues. A, During training, mice were given three 20 sec tone signals (solid horizontal bars) that coterminated with 1-sec-long electric shocks (0.5 mA; arrows). Both CD4-IgG- (n = 22) or EphA5-IgG-infused mice (n = 23) responded to training with increased freezing, a natural response to painful stimuli, and no significant differences were seen between groups. Relative duration (percentage; time per 60 sec) of freezing behavior is shown for 60 sec intervals. In addition to freezing, three other behavioral elements (bar graphs under line diagrams) are also shown. Note that fear correlates negatively with locomotion and grooming and positively with long-body. No significant differences were detected between the mice in any of the behavioral measures either before (interval 0–180 sec) or after (interval 180–360 sec) shock.B, A randomly assigned subset of trained mice (n = 16 for CD4-IgG-infused; _n_= 17 for EphA5-IgG-infused) was tested in the shock chamber for response to contextual stimuli. No tone cues or shocks were given. The freezing behavior (line diagram) of EphA5-IgG-infused mice was significantly impaired compared with that of the CD4-IgG-infused animals (F(1,31) = 24.926;p < 0.0001). In addition to freezing, relative duration of three other behavioral elements (bar graphs) is also shown for the entire session. EphA5-IgG-infused mice were found to exhibit an increased amount of locomotion (_t_ = 4.315; df = 31; _p_ < 0.0001) and grooming (_t_ = 2.133; df = 31; _p_ < 0.05) and exhibited decreased long-body posture (_t_= 2.100; df = 31; _p_ < 0.05), all suggesting decreased level of fear. _C_, A randomly assigned subset of trained mice (_n_ = 12 for CD4-IgG;_n_ = 13 for EphA5-IgG) was tested in the cued test. The cued test was conducted in a chamber that lacked the olfactory, visual, and tactile cues (the contextual stimuli) of the shock chamber. Mice received three tone signals alone (_solid horizontal bars_) but no shock. Both groups of mice responded to the tone cue with a robust increase in freezing (line diagram), and no significant differences were found between the two groups of mice on freezing (_F_(1,23) = 2.068;_p_ > 0.15) or any of the other behavioral measures (bar graphs) analyzed (t < 0.99; df = 23;_p_ > 0.30). Data obtained in fear conditioning are shown as mean ± SEM.
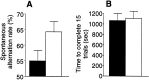
Fig. 6.
Infusion of ephrinA5-IgG in DBA/2 mice improves spontaneous alternation rate in the T-maze (T-CAT paradigm).A, EphrinA5-IgG-treated mice (white bar;n = 19) exhibited higher levels of alternation compared with CD4-IgG-infused mice (black bar;n = 17; t = 2.85; df = 34;p < 0.01). _B_, Time spent to complete 15 alternation trials did not differ between treatment groups (_t_ = 0.365; df = 34; _p_ > 0.710), suggesting that the improved alternation performance is not caused by motoric or motivational influences. Error bars represent SEM. Methods as in Figure 4 (also see Materials and Methods).
Fig. 7.
EphrinA5-IgG infusion significantly improves learning performance in a context-specific manner in fear conditioning in DBA/2 strain of mice. Methods are described in detail previously (Gerlai, 1998b). A, In the training session, no significant differences were found between ephrinA5-IgG-infused (white squares; n = 19 for training and tests) and CD4-IgG-infused (black circles;n = 17 for training and tests) mice in any of the behaviors (freezing: line diagram; other behavioral elements: bar graphs) measured, suggesting normal perceptual and motor performance.B, Response to contextual stimuli is shown. The freezing performance (line diagram) of ephrinA5-IgG-infused mice was significantly improved compared with the CD4-IgG-infused animals throughout the test session (F(1,34) = 33.434; p < 0.0001). In addition to freezing, increased level of fear in EphrinA5-IgG-infused mice is also indicated by changes in other behavioral elements (bar graphs). _C_, In the cued test, mice were placed in a chamber in which contextual stimuli were different from those of the shock chamber as explained in Figure 5_C_. The mice received three tone signals alone (_solid horizontal bars_) but no shock. All mice responded to the tone cue with a robust increase in freezing (line diagram), and no significant difference was found between the two groups of mice in freezing (_F_(1,34) = 0.004;_p_ > 0.95) or in any of the other behavioral measures (bar graphs) analyzed.
Fig. 8.
EphrinA5-IgG infusion significantly improves learning performance in a context-specific manner in fear conditioning in C57BL/6 mice. Methods are described in detail previously (Silva et al., 1996; Gerlai, 1998b). A, In the training session, no significant differences were found between ephrinA5-IgG-infused (black circles; n = 10 for training and tests) and CD4-IgG-infused (checkered squares;n = 10 for training and tests) mice in any of the behaviors measured (freezing: line diagram; other behavioral measures: bar graphs), suggesting normal perceptual and motor performance. Note that, to achieve a submaximal freezing response, only one tone (solid line) and shock (arrow) stimulus pairing was administered. B, Response to contextual stimuli is shown. The freezing performance (line diagram) of ephrinA5-IgG-infused mice was significantly improved compared with the CD4-IgG-infused animals throughout the test session (F(1,18) = 7.86; _p_= 0.01). In addition to freezing, increased level of fear in EphrinA5-IgG-infused mice is also indicated by decreased locomotion (t = 3.236; df = 1; p < 0.01), increased long-body posture (_t_ = 2.83; df = 1; _p_ = 0.01), and decreased grooming (_t_ = 2.024; df = 1; _p_ = 0.058) (bar graphs). _C_, In the cued test, mice were placed in a chamber in which contextual stimuli were different from those of the shock chamber as explained in Figure 5C. The mice received one tone signal alone (_solid horizontal bar_) but no shock. All mice responded to the tone cue with a robust increase in freezing (line diagram), and no significant difference was found between the two groups of mice in freezing (_F_(1,18) = 0.714; _p_> 0.40) or the other behavioral measures (bar graphs) analyzed except long-body, which remained slightly increased in ephrinA5-IgG-infused mice.
Fig. 9.
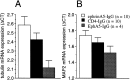
Transcriptional changes induced by immunoadhesins in two cytoskeletal proteins, tubulin (A) and MAP2 (B). No strain differences were found. The data (mean ± SEM) are pooled for the strains and are based on the number of PCR amplification cycles required to reach a threshold cleavage level of fluorescent reporter probe18, 19. Results normalized to GAPDH housekeeping gene transcript are shown (Δ_CT_). Sample sizes (n) are also indicated. Note that larger values represent smaller original mRNA amount in the hippocampal tissue sample analyzed. Note that expression of tubulin mRNA is increased by EphA5-IgG and decreased by ephrinA5-IgG infusion (ANOVA; F(2,21) = 5.02; p < 0.02). Expression changes in MAP2 show a similar but nonsignificant trend (ANOVA;_F_(2,21) = 1.75; _p_> 0.19).
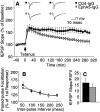
Fig. 10.
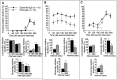
EphA5-IgG impairs LTP maintenance in hippocampal slices prepared from C57BL/6 mice. A, Field EPSP is expressed as percentage of baseline. Twenty sequential responses were averaged and plotted as one point. ANOVA revealed a significant immunoadhesin effect (F(1,8) = 8.09;p = 0.02) and a significant immunoadhesin × time interaction (F(29,232) = 4.98;p < 0.0001). Tukey's HSD test showed that the groups became significantly different (_p_ < 0.05) 90 min after tetanization. Representative traces before and after tetanization at corresponding time points, as indicated, are also shown. _B_, No immunoadhesin effect was observed on paired pulse facilitation (_F_(1,11) = 0.164;_p_ > 0.690), which was assessed by applying paired pulses of equivalent intensity at interpulse intervals as indicated. Facilitation ratios are calculated by expressing the slope of the second fEPSP as a percentage of the slope of the first fEPSP.C, Basal synaptic transmission, estimated by ratio of the fEPSP slope to the PSFV amplitude, was not altered by EphA5-IgG-infused (hatched bar) compared with CD4-IgG-infused (black bar) mice (_t_= 1.119; df = 14; p > 0.28). Estimation of basal synaptic transmission by I/O characteristics using Michaelis–Menten sigmoid curve fit revealed no significant differences (CD4-IgG, mean of 1.30 ± 0.368; EphA5-IgG, mean of 1.54 ± 0.219; p > 0.80).
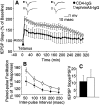
Fig. 11.
EphrinA5-IgG improves LTP and increases magnitude of PPF in hippocampal slices prepared from DBA/2. A, Field EPSP expressed as percentage of baseline. Twenty sequential responses were averaged and plotted as one point. ANOVA, including data from all time points, revealed an immunoadhesin effect that bordered significance (F(1,12) = 4.23;p = 0.06), whereas Tukey's HSD test showed that the groups were significantly different (p< 0.05) up to 260 min post-tetanization. Representative traces before and after tetanization at corresponding time points, as indicated, are also shown. _B_, PPF varied as a function of immunoadhesin treatment (_F_(1,21) =6.189;_p_ < 0.03), and no significant immunoadhesin × interval interaction (_F_(4,84) = 1.654; _p_ > 0.16) was seen. C, No significant differences were found in basal synaptic transmission between ephrinA5-IgG-infused (white bar) and CD4-IgG-infused (black bar) mice (_t_= 0.416; df = 18; p > 0.68). Estimation of basal synaptic transmission by I/O characteristics using Michaelis–Menten sigmoid curve fit revealed no significant differences (CD4-IgG, mean of 2.49 ± 0.33; ephrinA5-IgG, mean of 2.80 ± 0.97; p > 0.70).
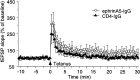
Fig. 12.
Chronic ephrinA5-IgG infusion leads to an apparent increase of LTP in hippocampal slices prepared from C57BL/6 mice. Individual responses measured once every 30 sec are plotted.Open circles, EphrinA5-IgG infusion (n = 6); filled triangles, CD4-IgG infusion (n = 6). One hippocampal slice per experimental animal was tested; thus, n represents the number of animals analyzed. Error bars indicate SE. ANOVA revealed that the apparent immunoadhesin effect was not significant (F(1,10) = 1.973; _p_= 0.190) nor was the time × immunoadhesin interaction significant (F(60,600) = 0.447;p > 0.50).
Similar articles
- Anesthesia induced retrograde amnesia is ameliorated by ephrinA5-IgG in mice: EphA receptor tyrosine kinases are involved in mammalian memory.
Gerlai R, McNamara A. Gerlai R, et al. Behav Brain Res. 2000 Mar;108(2):133-43. doi: 10.1016/s0166-4328(99)00139-4. Behav Brain Res. 2000. PMID: 10701657 - Regulation of hippocampal synaptic plasticity by the tyrosine kinase receptor, REK7/EphA5, and its ligand, AL-1/Ephrin-A5.
Gao WQ, Shinsky N, Armanini MP, Moran P, Zheng JL, Mendoza-Ramirez JL, Phillips HS, Winslow JW, Caras IW. Gao WQ, et al. Mol Cell Neurosci. 1998 Aug;11(5-6):247-59. doi: 10.1006/mcne.1998.0696. Mol Cell Neurosci. 1998. PMID: 9698392 - Ephrin-A binding and EphA receptor expression delineate the matrix compartment of the striatum.
Janis LS, Cassidy RM, Kromer LF. Janis LS, et al. J Neurosci. 1999 Jun 15;19(12):4962-71. doi: 10.1523/JNEUROSCI.19-12-04962.1999. J Neurosci. 1999. PMID: 10366629 Free PMC article. - Signaling by Eph receptors and their ephrin ligands.
Brückner K, Klein R. Brückner K, et al. Curr Opin Neurobiol. 1998 Jun;8(3):375-82. doi: 10.1016/s0959-4388(98)80064-0. Curr Opin Neurobiol. 1998. PMID: 9687349 Review. - [Molecular mechanisms underlying the formation of the topographic retinotectal map].
Yuasa-Kawada J, Noda M. Yuasa-Kawada J, et al. Tanpakushitsu Kakusan Koso. 2000 Feb;45(3 Suppl):307-15. Tanpakushitsu Kakusan Koso. 2000. PMID: 10707635 Review. Japanese. No abstract available.
Cited by
- Eph receptor and ephrin signaling in developing and adult brain of the honeybee (Apis mellifera).
Vidovic M, Nighorn A, Koblar S, Maleszka R. Vidovic M, et al. Dev Neurobiol. 2007 Feb 1;67(2):233-51. doi: 10.1002/dneu.20341. Dev Neurobiol. 2007. PMID: 17443785 Free PMC article. - A role for ephrin-A5 in axonal sprouting, recovery, and activity-dependent plasticity after stroke.
Overman JJ, Clarkson AN, Wanner IB, Overman WT, Eckstein I, Maguire JL, Dinov ID, Toga AW, Carmichael ST. Overman JJ, et al. Proc Natl Acad Sci U S A. 2012 Aug 14;109(33):E2230-9. doi: 10.1073/pnas.1204386109. Epub 2012 Jul 25. Proc Natl Acad Sci U S A. 2012. PMID: 22837401 Free PMC article. - Repetitive grooming and sensorimotor abnormalities in an ephrin-A knockout model for Autism Spectrum Disorders.
Wurzman R, Forcelli PA, Griffey CJ, Kromer LF. Wurzman R, et al. Behav Brain Res. 2015 Feb 1;278:115-28. doi: 10.1016/j.bbr.2014.09.012. Epub 2014 Oct 2. Behav Brain Res. 2015. PMID: 25281279 Free PMC article. - Ephrin-A5 regulates inter-male aggression in mice.
Sheleg M, Yochum CL, Richardson JR, Wagner GC, Zhou R. Sheleg M, et al. Behav Brain Res. 2015 Jun 1;286:300-7. doi: 10.1016/j.bbr.2015.03.001. Epub 2015 Mar 6. Behav Brain Res. 2015. PMID: 25746458 Free PMC article. - Activation of EphA receptors mediates the recruitment of the adaptor protein Slap, contributing to the downregulation of N-methyl-D-aspartate receptors.
Semerdjieva S, Abdul-Razak HH, Salim SS, Yáñez-Muñoz RJ, Chen PE, Tarabykin V, Alifragis P. Semerdjieva S, et al. Mol Cell Biol. 2013 Apr;33(7):1442-55. doi: 10.1128/MCB.01618-12. Epub 2013 Feb 4. Mol Cell Biol. 2013. PMID: 23382070 Free PMC article.
References
- Abeliovich A, Paylor R, Chen C, Krim J, Wehner J, Tonegawa S. PKCγ mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. - PubMed
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 1997;52:303–323. - PubMed
- Aiba A, Chen C, Herrup K, Rosenmund C, Stevens C, Tonegawa S. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell. 1994;79:365–375. - PubMed
- Bach ME, Hawkins RD, Osman M, Kandel ER, Mayford M. Impairment of spatial but not contextual memory in CaMKII mutant mice with a selective loss of hippocampal LTP in the range of the theta frequency. Cell. 1995;81:905–915. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources