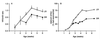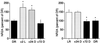Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development - PubMed (original) (raw)
Bidirectional, experience-dependent regulation of N-methyl-D-aspartate receptor subunit composition in the rat visual cortex during postnatal development
E M Quinlan et al. Proc Natl Acad Sci U S A. 1999.
Abstract
In the visual cortex, as elsewhere, N-methyl-D-aspartate receptors (NMDARs) play a critical role in triggering long-term, experience-dependent synaptic plasticity. Modifications of NMDAR subunit composition alter receptor function, and could have a large impact on the properties of synaptic plasticity. We have used immunoblot analysis to investigate the effects of age and visual experience on the expression of different NMDAR subunits in synaptoneurosomes prepared from rat visual cortices. NMDARs at birth are comprised of NR2B and NR1 subunits, and, over the first 5 postnatal weeks, there is a progressive inclusion of the NR2A subunit. Dark rearing from birth attenuates the developmental increase in NR2A. Levels of NR2A increase rapidly (in <2 hr) when dark-reared animals are exposed to light, and decrease gradually over the course of 3 to 4 days when animals are deprived of light. These data reveal that NMDAR subunit composition in the visual cortex is remarkably dynamic and bidirectionally regulated by sensory experience. We propose that NMDAR subunit regulation is a mechanism for experience-dependent modulation of synaptic plasticity in the visual cortex, and serves to maintain synaptic strength within an optimal dynamic range.
Figures
Figure 1
The synaptoneurosome fraction is enriched for synaptic profiles. (Left) Electron micrograph of a synaptoneurosome prepared from the visual cortex of a postnatal day-23 rat. (60,000×.) (Right) Representative immunoblot for the NMDAR proteins NR2A and NR2B. Synaptoneurosomes prepared from a postnatal day-23 visual cortex were resolved on a 12% polyacrylamide gel, transferred to nitrocellulose, and probed with anti-NR2A or anti-NR2B polyclonal antibodies and then with a horseradish peroxidase-coupled, anti-rabbit, secondary antibody. Visualization of immunoreactive bands was produced by enhanced chemiluminescence and captured on autoradiography film. Arrows indicate the positions of molecular weight markers (× 10−3).
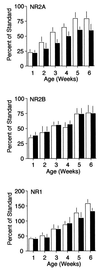
Figure 2
NMDAR subunit composition is regulated by age and visual experience. NR2A levels (Top) increase over development in both LR (white bars) and DR animals (dark bars), reaching an adult plateau at the age of 5 weeks. The level of NR2A protein is significantly reduced in synaptoneurosomes from the visual cortices of dark-reared animals (two-way ANOVA, P < 0.5, _n_ = 160). NR2B levels (_Middle_) also increase over development in both LR and DR animals, and reach an adult plateau at the age of 5 weeks. However, dark rearing does not significantly affect the complement of NR2B protein in synaptoneurosomes from the visual cortex (two-way ANOVA, _P_ > 0.1). NR1 levels (Bottom) increase over development in both LR and DR animals, and are not significantly reduced in synaptoneurosomes from the visual cortices of dark-reared animals (two-way ANOVA, P > 0.1).
Figure 3
The NR2A/B ratio correlates with the duration of NMDAR-mediated EPSCs. (A) The developmental increase in the NR2A/B ratio is attenuated in DR animals. (B) The developmental decrease in the duration of NMDAR-mediated EPSCs is attenuated in DR animals [data are replotted from Carmignoto and Vicini (3)]. The decay of the NMDAR-mediated EPSC, recorded in layer-IV neurons, was described by a double exponential function with a fast and a slow component. Over the course of development, the duration of NMDAR-mediated EPSCs progressively decreases, evident by the increased contribution of the fast component to the total NMDAR EPSC.
Figure 4
Experience-dependent regulation of NMDAR composition is reversible. (Left) Brief light exposure (2 hr) of DR animals induced a significant increase in NR2A protein that could be reversed by 72 to 96 hr of subsequent visual deprivation [one-way ANOVA, F (3, 18) = 13.14, P = 0.0004. Asterisks (*) denote significant difference vs. dark-reared controls in Student–Newman–Keuls posthoc comparison, P < 0.05)]. (Right) Placing LR animals in the dark for 72 to 96 hr, but not 24 hr, induced a decrease in the level of NR2A protein [one-way ANOVA, F (3, 24) = 6.49, P = 0.0023; asterisks (*) denote significant difference vs. light-reared controls in Student–Newman–Keuls posthoc comparison, P < 0.05)].
Similar articles
- Postnatal development of NR1, NR2A and NR2B immunoreactivity in the visual cortex of the rat.
Cao Z, Lickey ME, Liu L, Kirk E, Gordon B. Cao Z, et al. Brain Res. 2000 Mar 17;859(1):26-37. doi: 10.1016/s0006-8993(99)02450-6. Brain Res. 2000. PMID: 10720612 - Experience-dependent regulation of NMDA receptor subunit composition and phosphorylation in the retina and visual cortex.
Giannakopoulos M, Kouvelas ED, Mitsacos A. Giannakopoulos M, et al. Invest Ophthalmol Vis Sci. 2010 Apr;51(4):1817-22. doi: 10.1167/iovs.09-4438. Epub 2009 Oct 22. Invest Ophthalmol Vis Sci. 2010. PMID: 19850826 - Retinal influences induce bidirectional changes in the kinetics of N-methyl-D-aspartate receptor-mediated responses in striate cortex cells during postnatal development.
Olavarria JF, van Brederode JF, Spain WJ. Olavarria JF, et al. Neuroscience. 2007 Sep 7;148(3):683-99. doi: 10.1016/j.neuroscience.2007.07.005. Epub 2007 Jul 12. Neuroscience. 2007. PMID: 17706364 - [The developmental switch of visual cortex NMDA receptor NR2 subunits and its implications for visual plasticity].
Guo Y, Zhao K. Guo Y, et al. Zhonghua Yan Ke Za Zhi. 2015 Jun;51(6):470-6. Zhonghua Yan Ke Za Zhi. 2015. PMID: 26310123 Review. Chinese. - Making of a Synapse: Recurrent Roles of Drebrin A at Excitatory Synapses Throughout Life.
Aoki C, Sherpa AD. Aoki C, et al. Adv Exp Med Biol. 2017;1006:119-139. doi: 10.1007/978-4-431-56550-5_8. Adv Exp Med Biol. 2017. PMID: 28865018 Review.
Cited by
- Experience-dependent homeostatic synaptic plasticity in neocortex.
Whitt JL, Petrus E, Lee HK. Whitt JL, et al. Neuropharmacology. 2014 Mar;78:45-54. doi: 10.1016/j.neuropharm.2013.02.016. Epub 2013 Mar 4. Neuropharmacology. 2014. PMID: 23466332 Free PMC article. Review. - Defective Age-Dependent Metaplasticity in a Mouse Model of Alzheimer's Disease.
Megill A, Tran T, Eldred K, Lee NJ, Wong PC, Hoe HS, Kirkwood A, Lee HK. Megill A, et al. J Neurosci. 2015 Aug 12;35(32):11346-57. doi: 10.1523/JNEUROSCI.5289-14.2015. J Neurosci. 2015. PMID: 26269641 Free PMC article. - Glutamate receptor exocytosis and spine enlargement during chemically induced long-term potentiation.
Kopec CD, Li B, Wei W, Boehm J, Malinow R. Kopec CD, et al. J Neurosci. 2006 Feb 15;26(7):2000-9. doi: 10.1523/JNEUROSCI.3918-05.2006. J Neurosci. 2006. PMID: 16481433 Free PMC article. - Deletion of the NMDA receptor GluN2A subunit significantly decreases dendritic growth in maturing dentate granule neurons.
Kannangara TS, Bostrom CA, Ratzlaff A, Thompson L, Cater RM, Gil-Mohapel J, Christie BR. Kannangara TS, et al. PLoS One. 2014 Aug 1;9(8):e103155. doi: 10.1371/journal.pone.0103155. eCollection 2014. PLoS One. 2014. PMID: 25083703 Free PMC article. - Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity.
Yashiro K, Philpot BD. Yashiro K, et al. Neuropharmacology. 2008 Dec;55(7):1081-94. doi: 10.1016/j.neuropharm.2008.07.046. Epub 2008 Aug 8. Neuropharmacology. 2008. PMID: 18755202 Free PMC article. Review.
References
- Tsumoto T, Hagihara K, Sato H, Hata Y. Nature (London) 1987;327:513–514. - PubMed
- Carmignoto G, Vicini S. Science. 1992;258:1007–1011. - PubMed
- Roberts E B, Ramoa A S. J Neurophysiol. 1999;81:2587–2591. - PubMed
- Daw N W, Fox K, Sato H, Czepita D. J Neurophysiol. 1992;67:197–202. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources