Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus - PubMed (original) (raw)
Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus
M Frerking et al. Proc Natl Acad Sci U S A. 1999.
Abstract
Kainate (KA) receptor activation depresses stimulus-evoked gamma-aminobutyric acid (GABA-mediated) synaptic transmission onto CA1 pyramidal cells of the hippocampus and simultaneously increases the frequency of spontaneous GABA release through an increase in interneuronal spiking. To determine whether these two effects are independent, we examined the mechanism by which KA receptor activation depresses the stimulus-evoked, inhibitory postsynaptic current (IPSC). Bath application of the alpha-amino-3-hydroxy-5-methyl-4-isoxazole proprionic acid (AMPA)/KA receptor agonist KA in the presence of the AMPA receptor antagonist GYKI 53655 caused a large increase in spontaneous GABA release and a coincident depression of the evoked IPSC. The depressant action on the evoked IPSC was reduced, but not abolished, by the GABA(B) receptor antagonist SCH 50911, suggesting that the KA-induced increase in spontaneous GABA release depresses the evoked IPSC through activation of presynaptic GABA(B) receptors. KA had no resolvable effect on the potassium-induced increase in miniature IPSC frequency, suggesting that KA does not act through a direct effect on the release machinery or presynaptic calcium influx. KA caused a decrease in pyramidal cell input resistance, which was reduced by GABA(A) receptor antagonists. KA also caused a reduction in the size of responses to iontophoretically applied GABA, which was indistinguishable from the SCH 50911-resistant, residual depression of the evoked IPSC. These results suggest that KA receptor activation depresses the evoked IPSC indirectly by increasing interneuronal spiking and GABA release, leading to activation of presynaptic GABA(B) receptors, which depress GABA release, and postsynaptic GABA(A) receptors, which increase passive shunting.
Figures
Figure 1
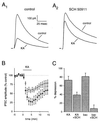
The KA-induced depression of eIPSC amplitude is reduced by the GABAB receptor antagonist SCH 50911. (A) Representative examples of the depression of the eIPSC induced by 10 μM KA in the absence (A1) or presence (A2) of the GABAB receptor antagonist SCH 50911 (20 μM). eIPSCs (n = 20) were averaged together in each of the displayed traces. (B) On average, KA reduced eIPSC amplitude to a greater extent in the absence of SCH 50911 (●) than in its presence (○). (C) The depressant action of KA is significantly reduced by SCH 50911, but a residual action remains. In contrast, the depressant action of the GABAB receptor agonist baclofen (10 μM) is abolished by SCH 50911.
Figure 2
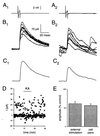
The KA-induced depression of the eIPSC is not dependent on depolarization of the presynaptic cell. (A) Consecutive current traces for a representative interneuron held in voltage clamp at −70 mV, with a brief depolarizing step to induce a single action potential, shown before (A1) and during (A2) the application of 10 μM KA. (B) Consecutive current traces for the pyramidal cell connected to the interneuron shown in A, showing IPSCs evoked by the presynaptic depolarizing step before (B1) and during (B2) KA application. Note the large increase in spontaneous IPSCs after KA application (B2). (C) Averages of the IPSCs shown in B, before (C1) and during (C2) the KA application. (D) Amplitude measurements from a different cell pair with a weaker connection, showing successes and failures of synaptic transmission before KA addition and a decrease in uIPSC amplitude upon KA application. The noise level increases dramatically upon KA application, presumably due to the increase in sIPSCs, preventing resolution of successes and failures during the KA application. (E) The SCH 50911-resistant depressant action of KA on eIPSCs is not significantly different from that on uIPSCs.
Figure 3
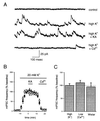
KA has no detectable effect on K+-evoked miniature IPSCs. (A) Representative current traces from an experiment in which the effect of KA on the frequency of K+-evoked mIPSCs was examined. (B) On average, K+-evoked mIPSC frequency was unaffected by KA application (●) when compared with control experiments in which KA was not added (○). (C) Summary of experiments on mIPSCs. mIPSC frequency was unaffected by KA when mIPSCs were evoked by high K+, by normal K+ in low Ca2+, or under normal conditions in Wistar rats.
Figure 4
The KA-induced increase in sIPSC frequency alters postsynaptic passive membrane properties. (A) Eight consecutive current traces in response to a 5-mV-hyperpolarizing step are shown before (Top) and during (Middle) KA application, after subtraction of average holding current from each trace. Note the increased sIPSC activity in the presence of KA and the associated large change in holding current (_I_h). The averaged current response in KA shows a larger steady–state value (Bottom), indicating an increase in resting conductance. (B) The KA-induced increase in sIPSC frequency causes a decrease in input resistance that correlates with the decrease in eIPSC amplitude. (B1) The large increase in _I_h upon KA application (●) is reduced when the KA is applied in the presence of 2 μM bicuculline (○). (B2) The average KA-induced depression of input resistance as a function of time (●) is not significantly different from the average KA-induced depression of eIPSC amplitude as a function of time (○). (C) KA alters eIPSC kinetics. (C1) KA depresses the eIPSC. (C2) eIPSCs in C1 before and after KA application are shown after normalization to their peak amplitudes. (D) On average, KA reduces the half-width of IPSCs. Individual experiments (●) are shown.
Figure 5
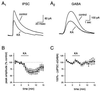
The KA-induced decrease in eIPSC amplitude in the presence of SCH 50911 is postsynaptic. (A) Average responses to synaptic stimulation (A1) or iontophoretic GABA application (A2) are shown before and during KA application. There are 15 sweeps included in each average. (B) The effects of KA on the eIPSC (●) are not significantly different from the effects of KA on the GABA response (○) in six cells in which the two responses were compared. (C) There is no significant action of KA on eIPSC amplitude in the presence of SCH 50911 once the postsynaptic effects are accounted for.
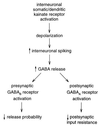
Figure 6
Model for the major actions of KA on the eIPSC. We propose that KA depresses the eIPSC mainly through a cascade in which somatic KA receptors depolarize interneurons, increasing the firing rate and thereby increasing GABA release. This GABA has two major effects: first, activation of presynaptic metabotropic GABAB receptors, and second, activation of postsynaptic ionotropic GABAA receptors. The GABAB receptors activate G protein-coupled signaling cascades, which depress the probability of release. The GABAA receptors decrease input resistance, causing a postsynaptic depression of synaptic signals. An additional minor action of KA on interneuronal fiber reliability remains a possibility (not shown).
Similar articles
- Kainate receptors depress excitatory synaptic transmission at CA3-->CA1 synapses in the hippocampus via a direct presynaptic action.
Frerking M, Schmitz D, Zhou Q, Johansen J, Nicoll RA. Frerking M, et al. J Neurosci. 2001 May 1;21(9):2958-66. doi: 10.1523/JNEUROSCI.21-09-02958.2001. J Neurosci. 2001. PMID: 11312279 Free PMC article. - Kindling enhances kainate receptor-mediated depression of GABAergic inhibition in rat granule cells.
Behr J, Gebhardt C, Heinemann U, Mody I. Behr J, et al. Eur J Neurosci. 2002 Sep;16(5):861-7. doi: 10.1046/j.1460-9568.2002.02152.x. Eur J Neurosci. 2002. PMID: 12372022 - Calcium channel involvement in GABAB receptor-mediated inhibition of GABA release in area CA1 of the rat hippocampus.
Doze VA, Cohen GA, Madison DV. Doze VA, et al. J Neurophysiol. 1995 Jul;74(1):43-53. doi: 10.1152/jn.1995.74.1.43. J Neurophysiol. 1995. PMID: 7472344 - In the developing hippocampus kainate receptors control the release of GABA from mossy fiber terminals via a metabotropic type of action.
Cherubini E, Caiati MD, Sivakumaran S. Cherubini E, et al. Adv Exp Med Biol. 2011;717:11-26. doi: 10.1007/978-1-4419-9557-5_2. Adv Exp Med Biol. 2011. PMID: 21713663 Review. - Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity.
Kullmann DM. Kullmann DM. Neuron. 2001 Nov 20;32(4):561-4. doi: 10.1016/s0896-6273(01)00507-4. Neuron. 2001. PMID: 11719198 Review.
Cited by
- Activation of presynaptic kainate receptors suppresses GABAergic synaptic transmission in the rat globus pallidus.
Jin XT, Smith Y. Jin XT, et al. Neuroscience. 2007 Oct 26;149(2):338-49. doi: 10.1016/j.neuroscience.2007.07.017. Epub 2007 Jul 20. Neuroscience. 2007. PMID: 17881134 Free PMC article. - Bidirectional modulation of GABA release by presynaptic glutamate receptor 5 kainate receptors in the basolateral amygdala.
Braga MF, Aroniadou-Anderjaska V, Xie J, Li H. Braga MF, et al. J Neurosci. 2003 Jan 15;23(2):442-52. doi: 10.1523/JNEUROSCI.23-02-00442.2003. J Neurosci. 2003. PMID: 12533604 Free PMC article. - Presynaptic cell dependent modulation of inhibition in cortical regions.
Ali AB. Ali AB. Curr Neuropharmacol. 2009 Jun;7(2):125-31. doi: 10.2174/157015909788848875. Curr Neuropharmacol. 2009. PMID: 19949571 Free PMC article. - Kainate receptors coming of age: milestones of two decades of research.
Contractor A, Mulle C, Swanson GT. Contractor A, et al. Trends Neurosci. 2011 Mar;34(3):154-63. doi: 10.1016/j.tins.2010.12.002. Epub 2011 Jan 20. Trends Neurosci. 2011. PMID: 21256604 Free PMC article. Review. - Astrocyte-mediated activation of neuronal kainate receptors.
Liu QS, Xu Q, Arcuino G, Kang J, Nedergaard M. Liu QS, et al. Proc Natl Acad Sci U S A. 2004 Mar 2;101(9):3172-7. doi: 10.1073/pnas.0306731101. Epub 2004 Feb 6. Proc Natl Acad Sci U S A. 2004. PMID: 14766987 Free PMC article.
References
- Ozawa S, Kamiya H, Tsuzuki K. Prog Neurobiol. 1998;54:581–618. - PubMed
- Hollmann M, Heinemann S. Annu Rev Neurosci. 1994;17:31–108. - PubMed
- Bettler B, Mulle C. Neuropharmacology. 1995;34:123–139. - PubMed
- Paternain A V, Morales M, Lerma J. Neuron. 1995;14:185–189. - PubMed
- Castillo P E, Malenka R C, Nicoll R A. Nature (London) 1997;388:182–186. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous