Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans - PubMed (original) (raw)
Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans
D Signor et al. J Cell Biol. 1999.
Abstract
The heterotrimeric motor protein, kinesin-II, and its presumptive cargo, can be observed moving anterogradely at 0.7 microm/s by intraflagellar transport (IFT) within sensory cilia of chemosensory neurons of living Caenorhabditis elegans, using a fluorescence microscope-based transport assay (Orozco, J.T., K.P. Wedaman, D. Signor, H. Brown, L. Rose, and J.M. Scholey. 1999. Nature. 398:674). Here, we report that kinesin-II, and two of its presumptive cargo molecules, OSM-1 and OSM-6, all move at approximately 1.1 microm/s in the retrograde direction along cilia and dendrites, which is consistent with the hypothesis that these proteins are retrieved from the distal endings of the cilia by a retrograde transport pathway that moves them along cilia and then dendrites, back to the neuronal cell body. To test the hypothesis that the minus end-directed microtubule motor protein, cytoplasmic dynein, drives this retrograde transport pathway, we visualized movement of kinesin-II and its cargo along dendrites and cilia in a che-3 cytoplasmic dynein mutant background, and observed an inhibition of retrograde transport in cilia but not in dendrites. In contrast, anterograde IFT proceeds normally in che-3 mutants. Thus, we propose that the class DHC1b cytoplasmic dynein, CHE-3, is specifically responsible for the retrograde transport of the anterograde motor, kinesin-II, and its cargo within sensory cilia, but not within dendrites.
Figures
Figure 1
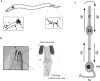
(a) Schematic representation of the amphid and phasmid chemosensory neurons indicating the regions monitored for in vivo transport of GFP fusion proteins. Shown is a simplified view of an adult wild-type worm with amphid neuron cell bodies and corresponding dendrites in the head, and phasmid neuron cell bodies and corresponding dendrites in the tail. Insets show a close-up view of the areas monitored for bidirectional IFT transport in ciliated endings (solid box), and bidirectional dendritic transport (dashed box). (b) Diagram showing high magnification view of an amphid sensillum. The diagram represents a transverse section through the amphid channel, which is located at the tip of the head, and normally contains eight ciliated amphid neurons that are open to the external environment through a pore in the cuticle (five are shown for simplicity; adapted from Perkins et al. 1986). (c) High magnification view of a single ciliated amphid neuron demonstrating the four classes of transport being studied: (1) anterograde intradendritic transport from the cell body toward the transition zone of the sensory cilium; (2) anterograde intraflagellar transport from the base of the transition zone toward the tip of the cilium; (3) retrograde intraflagellar transport from the tip of the cilium back toward the transition zone; and (4) retrograde intradendritic transport from the base of the transition zone back toward the cell body. For all: shown are the transition zones (TZ) and sensory cilia (C) that comprise the terminal endings of these chemosensory neurons, and the cell bodies (CB) and corresponding dendrites (D). Also shown in b are the cuticle (Cu) and Socket (So) and Sheath (Sh) supporting cells, and in c, the cell body (CB) and axon (Ax).
Figure 2
Rescue of the osm-1 mutant phenotype with the OSM-1::GFP fusion construct. Rescue of the osm-1 mutant phenotype is demonstrated by the ability of wild-type and transgenic worms to fill with the fluorescent dye DiI. osm-1 mutant worms are unable to dye fill because of structural defects in their amphid neuron sensory cilia. Top two rows demonstrate dye filling of the amphid chemosensory neurons of the head and corresponding DIC image; bottom two rows demonstrate dye filling of the phasmid chemosensory neurons of the tail and corresponding DIC image. Bar, 10 μm.
Figure 4
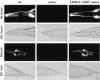
Retrograde IFT transport of kinesin-II and presumptive cargo molecules in sensory cilia. Worms expressing OSM-1::GFP, OSM-6::GFP, and KAP::GFP were assayed for retrograde transport in sensory cilia using methods discussed in Fig. 3. The retrograde movement of these molecules is seen as the movement of fluorescent particles from the tip of the sensory cilium toward the transition zone. Arrows point to fixed areas of fluorescence in the transition zones, arrowheads point to moving GFP particles. Bar, 5 μm.
Figure 3
Anterograde IFT transport of kinesin-II and presumptive cargo molecules in chemosensory cilia. Transgenic lines of worms expressing translational fusions of OSM-1, OSM-6, and KAP fused to GFP were assayed for anterograde IFT transport in vivo using time-lapse fluorescence microscopy. OSM-1::GFP, OSM-6::GFP, and KAP::GFP all accumulate in the transition zones at the base of the sensory cilia, and IFT rafts (GFP particles) are seen moving outward from these zones toward the tip of the cilium. Arrows point to fixed areas of fluorescence in the transition zones, arrowheads point to moving GFP particles. DIC images of a generic wild-type worm are provided for orientation, with arrowheads pointing to areas corresponding to the transition zones, and arrows demonstrating direction of movement. Bar, 5 μm.
Figure 5
Bidirectional dendritic transport of kinesin-II and presumptive cargo molecules in sensory neurons. The anterograde and retrograde transport of OSM-1::GFP, OSM-6::GFP, and KAP::GFP were assayed in amphid chemosensory neuron dendrites in vivo as the movement of GFP particles toward the transition zone and sensory cilia or toward the cell body, respectively. All three fusion proteins demonstrate bidirectional transport in amphid neuron dendrites. Anterograde transport of OSM-1::GFP and KAP::GFP are shown in the top two rows, with the transition zones and sensory cilia located anteriorly; retrograde transport of OSM-6::GFP is demonstrated in the bottom row, with the associated cell body located posteriorly. Arrows point to fixed regions along the dendrite for orientation, arrowheads point to moving GFP particles. Bar, 5 μm.
Figure 6
Mislocalization of OSM-6 and KAP in the cytoplasmic dynein mutant che-3. Homozygous che-3 mutant worms expressing OSM-6::GFP and KAP::GFP were examined for fluorescence localization by confocal microscopy. Arrows point to regions corresponding to the transition zones (TZ) and sensory cilia (C). (a, top row) OSM-6::GFP shows normal accumulation in the transition zones of the amphid chemosensory neurons and diffuse fluorescence along the sensory cilia in wild-type worms and in the osm-3 mutant background, but accumulates at the tips of the truncated sensory cilia in the che-3 mutant background. (bottom row) Similar defects in OSM-6::GFP localization are seen in phasmid chemosensory neurons of the tail, where OSM-6::GFP accumulates normally in the transition zones in wild-type and osm-3 mutant backgrounds, but accumulates abnormally as large fluorescent bulbs at the tips of the sensory cilia in worms lacking the CHE-3 cytoplasmic dynein. (b) KAP::GFP also accumulates abnormally at the tips of sensory cilia in amphid chemosensory neurons. Bar, 5 μm.
Figure 7
Retrograde transport of OSM-6::GFP occurs normally in dendrites lacking the CHE-3 cytoplasmic dynein. Homozygous che-3 mutants expressing the OSM-6::GFP fusion protein were examined for transport in sensory cilia and their corresponding dendrites. Shown are phasmid chemosensory neurons of the tail with an accumulation of OSM-6::GFP fusion protein at the tips of the sensory cilia (arrow), and diffuse protein along the corresponding dendrite back toward the cell body. Retrograde transport of OSM-6::GFP is defective in sensory cilia of che-3 mutants, but occurs normally in the corresponding dendrite (arrowheads). Bar, 5 μm.
Similar articles
- XBX-1 encodes a dynein light intermediate chain required for retrograde intraflagellar transport and cilia assembly in Caenorhabditis elegans.
Schafer JC, Haycraft CJ, Thomas JH, Yoder BK, Swoboda P. Schafer JC, et al. Mol Biol Cell. 2003 May;14(5):2057-70. doi: 10.1091/mbc.e02-10-0677. Epub 2003 Jan 26. Mol Biol Cell. 2003. PMID: 12802075 Free PMC article. - Intraflagellar transport: mechanisms of motor action, cooperation, and cargo delivery.
Prevo B, Scholey JM, Peterman EJG. Prevo B, et al. FEBS J. 2017 Sep;284(18):2905-2931. doi: 10.1111/febs.14068. Epub 2017 Apr 18. FEBS J. 2017. PMID: 28342295 Free PMC article. Review. - Analysis of intraflagellar transport in C. elegans sensory cilia.
Hao L, Acar S, Evans J, Ou G, Scholey JM. Hao L, et al. Methods Cell Biol. 2009;93:235-66. doi: 10.1016/S0091-679X(08)93013-2. Epub 2009 Dec 4. Methods Cell Biol. 2009. PMID: 20409821 - The retrograde IFT machinery of C. elegans cilia: two IFT dynein complexes?
Hao L, Efimenko E, Swoboda P, Scholey JM. Hao L, et al. PLoS One. 2011;6(6):e20995. doi: 10.1371/journal.pone.0020995. Epub 2011 Jun 10. PLoS One. 2011. PMID: 21695221 Free PMC article. - Intraflagellar transport motors in Caenorhabditis elegans neurons.
Scholey JM, Ou G, Snow J, Gunnarson A. Scholey JM, et al. Biochem Soc Trans. 2004 Nov;32(Pt 5):682-4. doi: 10.1042/BST0320682. Biochem Soc Trans. 2004. PMID: 15493987 Review.
Cited by
- TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia.
Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. Mukhopadhyay S, et al. Genes Dev. 2010 Oct 1;24(19):2180-93. doi: 10.1101/gad.1966210. Genes Dev. 2010. PMID: 20889716 Free PMC article. - Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella.
Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG. Pazour GJ, et al. J Cell Biol. 2000 Oct 30;151(3):709-18. doi: 10.1083/jcb.151.3.709. J Cell Biol. 2000. PMID: 11062270 Free PMC article. - Small GTPases and cilia.
Li Y, Hu J. Li Y, et al. Protein Cell. 2011 Jan;2(1):13-25. doi: 10.1007/s13238-011-1004-7. Epub 2011 Feb 20. Protein Cell. 2011. PMID: 21337006 Free PMC article. Review. - Intraflagellar transport dynein is autoinhibited by trapping of its mechanical and track-binding elements.
Toropova K, Mladenov M, Roberts AJ. Toropova K, et al. Nat Struct Mol Biol. 2017 May;24(5):461-468. doi: 10.1038/nsmb.3391. Epub 2017 Apr 10. Nat Struct Mol Biol. 2017. PMID: 28394326 Free PMC article. - The two motor domains of KIF3A/B coordinate for processive motility and move at different speeds.
Zhang Y, Hancock WO. Zhang Y, et al. Biophys J. 2004 Sep;87(3):1795-804. doi: 10.1529/biophysj.104.039842. Biophys J. 2004. PMID: 15345558 Free PMC article.
References
- Bargmann C.I., Thomas J.H., Horvitz H.R. Chemosensory cell function in the behaviour and development of C. elegans . Cold Spring Harbor Symp. Quant. Biol. 1990;55:529–538. - PubMed
- Burton P.R. Dendrites of mitral cell neurons contain microtubules of opposite polarity. Brain Res. 1988;473:107–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases