A major role for the rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea - PubMed (original) (raw)
A major role for the rho-associated coiled coil forming protein kinase in G-protein-mediated Ca2+ sensitization through inhibition of myosin phosphatase in rabbit trachea
K Iizuka et al. Br J Pharmacol. 1999 Oct.
Abstract
1 G protein-mediated Ca2+ sensitization of airway smooth muscle contraction was investigated with respect to the relative importance of Rho-associated coiled coil forming protein kinase (ROCK) and protein kinase C (PKC). We examined the effects of Y-27632, a ROCK inhibitor, and GF 109203X, a PKC inhibitor, on guanosine 5'-O-(3-thiotriphosphate) (GTPgammaS)-induced contraction in alpha-toxin- or beta-escin-permeabilized rabbit trachea. 2 Although pre-treatment with Y-27632 dose-dependently inhibited GTPgammaS (10 microM)-induced Ca2+ sensitization of alpha-toxin-permeabilized trachea, a Y-27632-insensitive component (approximately 16% of the maximum contraction) was retained during the early phase of the GTPgammaS response in the presence of Y-27632 (100 microM). 3 GF 109203X (5 microM) abolished 1 microM 4beta-phorbol 12, 13-dibutyrate (PDBu)-induced, but only partially inhibited the GTPgammaS-induced Ca2+ sensitization. A combination of Y-27632 (100 microM) and GF 109203X (5 microM) totally abolished the GTPgammaS response. 4 GTPgammaS caused only a small contraction in the absence of Ca2+. Wortmannin (30 microM), a myosin light chain kinase (MLCK) inhibitor, completely inhibited Ca2+-induced contraction. ATP-triggered contraction of the strip which had been treated with calyculin A (1 microM), a phosphatase inhibitor, in rigor solutions was markedly slowed by worthmannin (30 microM), but not by Y-27632 (100 microM), in the presence of GTPgammaS and Ca2+. 5 GTPgammaS, but not PDBu, contracted the beta-escin-permeabilized trachea in the absence of Ca2+, but the presence of Ca2+-independent MLCK. 6 We conclude that ROCK plays a primary role in G-protein-mediated Ca2+ sensitization, which requires MLCK activity, with minor contribution of PKC to the early phase of contraction, and PDBu utilizes conventional PKC(s) in airway smooth muscle.
Figures
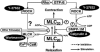
Figure 1
Putative mechanism of phosphorylation of mysoin light chain in smooth muscle. In ATP free (rigor) solutions Y-27632, wortmannin, and calyculin A inhibit ROCK, MLCK, and SMPP-1M, respectively, although ROCK, MLCK, and other kinase(s) cannot phosphorylate the substrates even when Ca2+ and GTPγS are present because ATP is absent. Once SMPP-1M has been inhibited by calyculin A, Rho/ROCK-mediated signalling cannot affect SMPP-1M activity. ATP application triggers phosphorylation of MLC20, resulting in contraction. The rate of force rise, but not the final amplitude, reflects ROCK, MLCK and other kinase(s) activities towards MLC20. CaM, calmodulin; MLC20, 20 kDa myosin light chain; MLCK, myosin light chain kinase; ROCK, Rho-associated coiled coil forming protein kinase; SMPP-1M, smooth muscle protein phosphatase 1 associated with myosin.
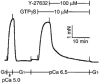
Figure 2
Effect of subsequent addition of Y-27632 on GTPγS-induced Ca2+ sensitization. When the GTPγS-induced Ca2+ sensitization reached a peak, a high concentration of Y-27632 was applied to the strip. The traces are representative of five experiments.
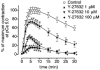
Figure 3
Time course of GTPγS-induced Ca2+ sensitization in the presence or absence of Y-27632. After stable pCa 5.0 response was obtained, the α-toxin-permeabilized tracheal strips were pre-incubated for 30 min without or with Y-27632 at indicated concentrations. When the submaximal contraction at pCa 6.5 became stable, GTPγS (10 μ
M
) was added to the strips, and the serial changes in tension were observed for 30 min. Y-27632 was present during GTPγS-induced Ca2+ sensitization. Developed force was normalized to the initial pCa 5.0 response. *P<0.05 vs control. (_n_=4–7).
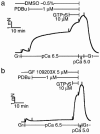
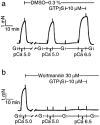
Figure 4
Complete inhibition of PDBu- but not GTPγS-induced Ca2+ sensitization by GF 109203X. After stable pCa 5.0 response was obtained, the α-toxin-permeabilized tracheal strips were preincubated for 30 min with either GF 109203X (5 μ
M
, b) or 0.5% dimethyl sulphoxide (DMSO, a). When the submaximal contraction at pCa 6.5 became stable, PDBu (1 μ
M
) was added to the strips, followed by application of GTPγS (10 μ
M
) as indicated. The traces are representative of four experiments.
Figure 5
Lack of effect of GF 109203X on Ca2+-induced contraction of Triton X-100-permeabilized trachea. The trachea was contracted by a solution of pCa 5.0 containing calmodulin (CaM, 5 μ
M
), and relaxed in a relaxing solution (G1). When the pCa 6.2-induced contraction became stable, GF 109203X followed by wortmannin were added to the strip. The trace was representative of four experiments. All solutions except for pCa 5.0 contained 1 μ
M
CaM.
Figure 6
GTPγS-induced Ca2+ sensitization in the presence of Y-27632, GF 109203X, or both. After stable pCa 5.0 response was obtained, the α-toxin-permeabilized tracheal strips were preincubated for 30 min without or with either GF 109203X, Y-27632, or both. When the submaximal contraction at pCa 6.5 became stable, GTPγS (10 μ
M
) was added to the strips, and the changes in tension were observed for 30 min. The reagents were present during GTPγS-induced Ca2+ sensitization, and control experiments were carried out in the presence of vehicle 0.5% dimethyl sulphoxide (DMSO), water, or both. Developed force was normalized to the initial pCa 5.0 response. *P<0.05 vs control. (_n_=4–11).
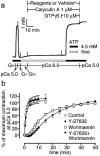
Figure 7
Complete inhibition of high Ca2+ alone and low Ca2+ plus GTPγS-induced contractions by wortmannin. After obtaining the pCa 5.0 response, the strips were treated with wortmannin (30 μ
M
) (b) or the vehicle (0.3% dimethyl sulphoxide, DMSO) (a) for 30 min in a relaxing solution (G1), followed by exposure of high Ca2+ (pCa 5.0) and its washing. Then, GTPγS (10 μ
M
) was added to the strips in G1, and quick transferring the strips to the pCa 6.5 solution containing GTPγS. The traces are representative of six experiments.
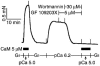
Figure 8
The effects of wortmannin and Y-27632 on ATP-triggered contraction of calyculin A-treated trachea. After the pCa 5.0 response was obtained, the α-toxin-permeabilized tracheal strips were incubated in an ATP free Ca2+ free solution (G10) for 10 min to remove ATP from the strip, and then incubated in a pCa 6.5 rigor solution containing calyculin A (1 μ
M
) for 60 min. GTPγS (10 μ
M
) was present as indicated. Contraction was initiated by addition of ATP (a). Time matched experiments were carried out in the presence of wortmannin (30 μ
M
), Y-27632 (100 μ
M
), or both 60 min before and during contraction as indicated*. Data are summarized in (b) (_n_=4–5).
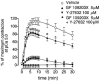
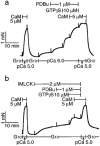
Figure 9
Requirement of MLCK activity but not Ca2+ itself for GTPγS-induced contraction in β-escin-permeabilized trachea. After stable pCa 5.0 plus 5 μ
M
calmodulin (CaM) response was obtained, the β-escin-permeabilized strips were contracted either by Ca2+-independent MLCK (IMLCK, 2 μ
M
) in G10 (buffered by 10 m
M
EGTA) (b) or by pCa 6.0 (a). PDBu (1 μ
M
), GTPγS (10 μ
M
), and CaM (5 μ
M
) were added sequentially to each strip as indicated. Unless noted otherwise all solutions contained CaM (0.1 μ
M
). Data are summarized in (c). Submax. contraction means either pCa 6.0- or IMLCK-induced force prior to addition of Ca2+ sensitizing agents. The responses to pCa 6.0, IMLCK, PDBu, GTPγS, and CaM were normalized to the initial maximum contraction of each strip. **P<0.01, (Mann-Whitney _U_-test, _n_=4). In separate set of experiments, Y-27632 was added to the peak of contraction induced by GTPγS in the presence of IMLCK, followed by application of calyculin A. The traces are representative of four experiments (d).
Figure 9
Requirement of MLCK activity but not Ca2+ itself for GTPγS-induced contraction in β-escin-permeabilized trachea. After stable pCa 5.0 plus 5 μ
M
calmodulin (CaM) response was obtained, the β-escin-permeabilized strips were contracted either by Ca2+-independent MLCK (IMLCK, 2 μ
M
) in G10 (buffered by 10 m
M
EGTA) (b) or by pCa 6.0 (a). PDBu (1 μ
M
), GTPγS (10 μ
M
), and CaM (5 μ
M
) were added sequentially to each strip as indicated. Unless noted otherwise all solutions contained CaM (0.1 μ
M
). Data are summarized in (c). Submax. contraction means either pCa 6.0- or IMLCK-induced force prior to addition of Ca2+ sensitizing agents. The responses to pCa 6.0, IMLCK, PDBu, GTPγS, and CaM were normalized to the initial maximum contraction of each strip. **P<0.01, (Mann-Whitney _U_-test, _n_=4). In separate set of experiments, Y-27632 was added to the peak of contraction induced by GTPγS in the presence of IMLCK, followed by application of calyculin A. The traces are representative of four experiments (d).
Similar articles
- Relaxation of contracted rabbit tracheal and human bronchial smooth muscle by Y-27632 through inhibition of Ca2+ sensitization.
Yoshii A, Iizuka K, Dobashi K, Horie T, Harada T, Nakazawa T, Mori M. Yoshii A, et al. Am J Respir Cell Mol Biol. 1999 Jun;20(6):1190-200. doi: 10.1165/ajrcmb.20.6.3441. Am J Respir Cell Mol Biol. 1999. PMID: 10340938 - The effects of the Rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPgammaS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle.
Fu X, Gong MC, Jia T, Somlyo AV, Somlyo AP. Fu X, et al. FEBS Lett. 1998 Nov 27;440(1-2):183-7. doi: 10.1016/s0014-5793(98)01455-0. FEBS Lett. 1998. PMID: 9862451 - 8-Bromo-cAMP decreases the Ca2+ sensitivity of airway smooth muscle contraction through a mechanism distinct from inhibition of Rho-kinase.
Endou K, Iizuka K, Yoshii A, Tsukagoshi H, Ishizuka T, Dobashi K, Nakazawa T, Mori M. Endou K, et al. Am J Physiol Lung Cell Mol Physiol. 2004 Oct;287(4):L641-8. doi: 10.1152/ajplung.00287.2003. Epub 2004 Apr 30. Am J Physiol Lung Cell Mol Physiol. 2004. PMID: 15121638 - Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II.
Somlyo AP, Somlyo AV. Somlyo AP, et al. J Physiol. 2000 Jan 15;522 Pt 2(Pt 2):177-85. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. J Physiol. 2000. PMID: 10639096 Free PMC article. Review. - Calcium sensitization mechanisms in detrusor smooth muscles.
Anjum I. Anjum I. J Basic Clin Physiol Pharmacol. 2018 Jun 27;29(3):227-235. doi: 10.1515/jbcpp-2017-0071. J Basic Clin Physiol Pharmacol. 2018. PMID: 29306925 Review.
Cited by
- Comparisons of actin filament disruptors and Rho kinase inhibitors as potential antiglaucoma medications.
Tian B, Kaufman PL. Tian B, et al. Expert Rev Ophthalmol. 2012 Apr;7(2):177-187. doi: 10.1586/eop.12.12. Expert Rev Ophthalmol. 2012. PMID: 22737177 Free PMC article. - RGD-Dependent Epithelial Cell-Matrix Interactions in the Human Intestinal Crypt.
Benoit YD, Groulx JF, Gagné D, Beaulieu JF. Benoit YD, et al. J Signal Transduct. 2012;2012:248759. doi: 10.1155/2012/248759. Epub 2012 Sep 5. J Signal Transduct. 2012. PMID: 22988499 Free PMC article. - Signaling and regulation of G protein-coupled receptors in airway smooth muscle.
Billington CK, Penn RB. Billington CK, et al. Respir Res. 2003;4(1):2. Epub 2003 Mar 14. Respir Res. 2003. PMID: 12648290 Free PMC article. Review. - The washout phenomenon in aqueous outflow--why does it matter?
Gong H, Freddo TF. Gong H, et al. Exp Eye Res. 2009 Apr;88(4):729-37. doi: 10.1016/j.exer.2009.01.015. Exp Eye Res. 2009. PMID: 19385044 Free PMC article. Review. - Rho-associated coiled-coil containing kinases (ROCK): structure, regulation, and functions.
Julian L, Olson MF. Julian L, et al. Small GTPases. 2014;5:e29846. doi: 10.4161/sgtp.29846. Epub 2014 Jul 10. Small GTPases. 2014. PMID: 25010901 Free PMC article. Review.
References
- AMANO M., CHIHARA K., KIMURA K., FUKATA Y., NAKAMURA N., MATSUURA Y., KAIBUCHI K. Formation of actin stress fibers and focal adhesions enhanced by Rho-kinase. Science. 1997;275:1308–1311. - PubMed
- AMANO M., ITO M., KIMURA K., FUKATA Y., CHIHARA K., NAKANO T., MATSUURA Y., KAIBUCHI K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J. Biol. Chem. 1996;271:20246–20249. - PubMed
- BREMERICH D.H., HIRASAKI A., JONES K.A., WARNER D.O. Halothane attenuation of calcium sensitivity in airway smooth muscle. Mechanisms of action during muscarinic receptor stimulation. Anesthesiology. 1997a;87:94–101. - PubMed
- BREMERICH D.H., WARNER D.O., LORENZ R.R., SHUMWAY R., JONES K.A. Role of protein kinase C in calcium sensitization during muscarinic stimulation in airway smooth muscle. Am. J. Physiol. 1997b;273:L775–L781. - PubMed
- FU X., GONG M.C., JIA T., SOMLYO A.V., SOMLYO A.P. The effects of the rho-kinase inhibitor Y-27632 on arachidonic acid-, GTPγS-, and phorbol ester-induced Ca2+-sensitization of smooth muscle. FEBS lett. 1998;440:183–187. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous