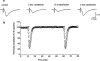Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices - PubMed (original) (raw)
Effect of A2A adenosine receptor stimulation and antagonism on synaptic depression induced by in vitro ischaemia in rat hippocampal slices
S Latini et al. Br J Pharmacol. 1999 Nov.
Abstract
1. In the present study we investigated the role of A2A adenosine receptors in hippocampal synaptic transmission under in vitro ischaemia-like conditions. 2. The effects of adenosine, of the selective A2A receptor agonist, CGS 21680 (2-[p-(2-carboxyethyl)-phenethylamino]-5'-N-ethylcarboxamidoade nos ine ), and of selective A2A receptor antagonists, ZM 241385 (4-(2-[7-amino-2-(2-furyl)-¿1,2,4¿-triazolo¿2,3-a¿¿1,3, 5¿triazin-5-ylamino]ethyl)phenol) and SCH 58261 (7-(2-phenylethyl)-5-amino-2-(2-furyl)-pyrazolo-[4,3-e]-1,2, 4-triazolo[1,5-c]pyrimidine), have been evaluated on the depression of field e.p.s.ps induced by an in vitro ischaemic episode. 3. The application of 2 min of in vitro ischaemia brought about a rapid and reversible depression of field e.p.s.ps, which was completely prevented in the presence of the A1 receptor antagonist DPCPX (1, 3-dipropyl-8-cyclopentylxanthine) (100 nM). On the other hand both A2A receptor antagonists, ZM 241385 and SCH 58261, by themselves did not modify the field e.p.s.ps depression induced by in vitro ischaemia. 4. A prolonged application of either adenosine (100 micronM) or CGS 21680 (30, 100 nM) before the in vitro ischaemic episode, significantly reduced the synaptic depression. These effects were antagonized in the presence of ZM 241385 (100 nM). 5. SCH 58261 (1 and 50 nM) did not antagonize the effect of 30 nM CGS 21680 on the ischaemia-induced depression. 6. These results indicate that in the CA1 area of the hippocampus the stimulation of A2A adenosine receptors attenuates the A1-mediated depression of synaptic transmission induced by in vitro ischaemia.
Figures
Figure 1
Modifications in the amplitude of synaptic potentials induced by 2 min of in vitro ischaemia. (a) Traces of field e.p.s.ps recorded during a typical experiment, under control conditions, at the end of 2 min of in vitro ischaemia and after 15 s and 5 min of reperfusion. (b) Time-course of field e.p.s.p. amplitude, expressed as per cent of baseline, before, during and after the application of two consecutive in vitro ischaemic insults of 2 min (indicated by bars on graph). Each value represents the mean±s.e.mean of six experiments. Absolute values (means±s.e.mean) of field e.p.s.p. amplitude in normoxic conditions (100%) were 1.06±0.04 mV before the first period of in vitro ischaemia and 1.07±0.05 mV before the second.
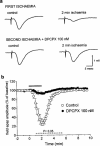
Figure 2
Effect of the A1 receptor antagonist DPCPX, on the field e.p.s.p. depression induced by 2 min of in vitro ischaemia. (a) Traces of field e.p.s.ps recorded during a typical experiment, under control conditions and at the end of 2 min of in vitro ischaemia both in the absence and in the presence of DPCPX. (b) Time-course of field e.p.s.p. amplitude modifications during the first and second ischaemic episodes. Field e.p.s.p. amplitude is expressed as the percentage of averaged potentials recorded before the respective ischaemic periods. Each value represents the mean±s.e.mean of three experiments. Absolute values (means±s.e.mean) of field e.p.s.p. amplitude in normoxic conditions (100%) were 0.92±0.06 mV before the first period of in vitro ischaemia and 1.01±0.07 mV (+10%, P<0.05) before the second, in the presence of DPCPX. The dotted line under the graph indicates the statistical significance of the effect of DPCPX, evaluated with the paired Students _t_-test at each time.
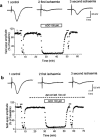
Figure 3
Effect of a prolonged application of adenosine on field e.p.s.p. depression induced by in vitro ischaemia. (a) Time-course of changes in field e.p.s.p. amplitude elicited by application of adenosine (100 μ
M
) in one typical of four experiments. Traces show field e.p.s.ps recorded at the time indicated by numbers in the graph. Adenosine was applied 15 min after the recovery of field e.p.s.p. amplitude from the first ischaemic insult, and was maintained for 20 min. The second ischaemic episode was applied immediately after recovery from adenosine effect. (b) Time-course of changes in field e.p.s.p. amplitude elicited by application of adenosine (100 μ
M
) in the presence of the A2A adenosine receptor antagonist, ZM 241385 in one typical of four experiments. Traces show field e.p.s.ps recorded at the time indicated by numbers in the graph. ZM 241385 (100 n
M
) was applied 15 min before adenosine and was maintained during the second ischaemic episode and until the end of the experiment. (a) and (b) are from different slices.
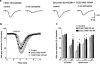
Figure 4
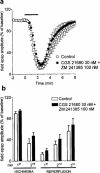
Effect of the selective A2A receptor agonist, CGS 21680, on the field e.p.s.p. depression induced by 2 min of in vitro ischaemia. CGS 21680 was applied 20 min before the second ischaemic period and maintained until the end of the experiment. (a) Traces of field e.p.s.ps recorded during a typical experiment, under normoxic and ischaemic conditions, either in the absence or presence of 100 n
M
CGS 21680. (b) Time course of field e.p.s.p. amplitude modifications and effect of two concentrations of CGS 21680 (30 and 100 n
M
). Field e.p.s.p. amplitude is expressed as the percentage of averaged potentials recorded before the respective ischaemic periods. Since no significant differences were found between the first ischaemic depression in the group of experiments with 30 n
M
and in the group with 100 n
M
CGS 21680, the values from these groups are shown in the figure averaged and compared with those obtained during the second ischaemic depression in the presence of CGS 21680. Each value represents the mean±s.e.mean of 12 experiments for control, seven experiments for 30 n
M
CGS 21680 and five experiments for 100 n
M
CGS 21680. (c) Each bar represents the average amplitude of four consecutive field e.p.s.ps (1 min), recorded during the first and second minute of in vitro ischaemia and during the first, second and third minute of reperfusion (expressed as per cent of controls). Differences among data were analysed by ANOVA (P<0.001) followed by post hoc Fisher's test: *P<0.05 vs control, °P<0.05 vs 30 n
M
CGS 21680.
Figure 5
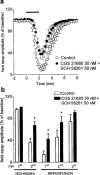
Effect of the A2A adenosine receptor antagonist ZM 241385 on the CGS 21680-induced reduction of in vitro ischaemic depression. (a) 100 n
M
ZM 241385 was applied 20 min before 30 n
M
CGS 21680 and maintained during the second period of in vitro ischaemia and until the end of the experiment. Field e.p.s.p. amplitude is expressed as the percentage of averaged potentials recorded before the respective ischaemic periods. Each value represents the mean±s.e.mean of five experiments. Averaged (means±s.e.mean) field e.p.s.p. amplitudes in normoxic conditions (100%) were: 1.13±0.06 before the first period of ischaemia and 1.18±0.06 before the second, in the presence of drugs. (b) Each bar represents the average amplitude of four consecutive field e.p.s.ps recorded during the first and second minute of in vitro ischaemia and during the first, second and third minute of reperfusion. No statistically significant differences among data were observed by the application of ANOVA followed by post hoc Fisher's test.
Figure 6
Effect of the A2A adenosine receptor antagonist SCH 58261 on the CGS 21680-induced reduction in in vitro ischaemic depression. (a) 50 n
M
SCH 58261 was applied 20 min before 30 n
M
CGS 21680 and maintained during the second period of in vitro ischaemia and until the end of the experiment. Field e.p.s.p. amplitude is expressed as the percentage of averaged potentials recorded before the respective ischaemic periods. Each value represents the mean±s.e.mean of five experiments. Averaged (means±s.e.mean) field e.p.s.p. amplitudes in normoxic conditions (100%) were: 0.98±0.02 mV before the first period of in vitro ischaemia and 1.02±0.03 before the second, in the presence of drugs. (b) Each bar represents the average amplitude of four consecutive field e.p.s.ps recorded during the first and second minute of in vitro ischaemia and during the first, second and third minute of reperfusion. Differences among data were analysed by ANOVA (P<0.001) followed by post hoc Fisher's test: *P<0.05 vs control.
Similar articles
- Brief, repeated, oxygen-glucose deprivation episodes protect neurotransmission from a longer ischemic episode in the in vitro hippocampus: role of adenosine receptors.
Pugliese AM, Latini S, Corradetti R, Pedata F. Pugliese AM, et al. Br J Pharmacol. 2003 Sep;140(2):305-14. doi: 10.1038/sj.bjp.0705442. Epub 2003 Aug 11. Br J Pharmacol. 2003. PMID: 12970092 Free PMC article. - Cross talk between A(1) and A(2A) adenosine receptors in the hippocampus and cortex of young adult and old rats.
Lopes LV, Cunha RA, Ribeiro JA. Lopes LV, et al. J Neurophysiol. 1999 Dec;82(6):3196-203. doi: 10.1152/jn.1999.82.6.3196. J Neurophysiol. 1999. PMID: 10601453 - Adenosine A(2A) receptor facilitation of hippocampal synaptic transmission is dependent on tonic A(1) receptor inhibition.
Lopes LV, Cunha RA, Kull B, Fredholm BB, Ribeiro JA. Lopes LV, et al. Neuroscience. 2002;112(2):319-29. doi: 10.1016/s0306-4522(02)00080-5. Neuroscience. 2002. PMID: 12044450 - Selective adenosine A2A receptor antagonists.
Ongini E, Monopoli A, Cacciari B, Baraldi PG. Ongini E, et al. Farmaco. 2001 Jan-Feb;56(1-2):87-90. doi: 10.1016/s0014-827x(01)01024-2. Farmaco. 2001. PMID: 11347973 Review. - Purines and neuroprotection.
Stone TW. Stone TW. Adv Exp Med Biol. 2002;513:249-80. doi: 10.1007/978-1-4615-0123-7_9. Adv Exp Med Biol. 2002. PMID: 12575824 Review.
Cited by
- Smoke extract impairs adenosine wound healing: implications of smoke-generated reactive oxygen species.
Allen-Gipson DS, Zimmerman MC, Zhang H, Castellanos G, O'Malley JK, Alvarez-Ramirez H, Kharbanda K, Sisson JH, Wyatt TA. Allen-Gipson DS, et al. Am J Respir Cell Mol Biol. 2013 May;48(5):665-73. doi: 10.1165/rcmb.2011-0273OC. Am J Respir Cell Mol Biol. 2013. PMID: 23371060 Free PMC article. - AMP N(1)-oxide, a unique compound of royal jelly, induces neurite outgrowth from PC12 cells via signaling by protein kinase A independent of that by mitogen-activated protein kinase.
Hattori N, Nomoto H, Fukumitsu H, Mishima S, Furukawa S. Hattori N, et al. Evid Based Complement Alternat Med. 2010 Mar;7(1):63-8. doi: 10.1093/ecam/nem146. Epub 2007 Oct 29. Evid Based Complement Alternat Med. 2010. PMID: 18955270 Free PMC article. - The role of ATP and adenosine in the brain under normoxic and ischemic conditions.
Pedata F, Melani A, Pugliese AM, Coppi E, Cipriani S, Traini C. Pedata F, et al. Purinergic Signal. 2007 Sep;3(4):299-310. doi: 10.1007/s11302-007-9085-8. Epub 2007 Oct 11. Purinergic Signal. 2007. PMID: 18404443 Free PMC article. - Role of adenosine A3 receptors on CA1 hippocampal neurotransmission during oxygen-glucose deprivation episodes of different duration.
Pugliese AM, Coppi E, Volpini R, Cristalli G, Corradetti R, Jeong LS, Jacobson KA, Pedata F. Pugliese AM, et al. Biochem Pharmacol. 2007 Sep 1;74(5):768-79. doi: 10.1016/j.bcp.2007.06.003. Epub 2007 Jun 7. Biochem Pharmacol. 2007. PMID: 17626785 Free PMC article. - Pharmacological Characterization of P626, a Novel Dual Adenosine A2A/A2B Receptor Antagonist, on Synaptic Plasticity and during an Ischemic-like Insult in CA1 Rat Hippocampus.
Venturini M, Cherchi F, Santalmasi C, Frulloni L, Dettori I, Catarzi D, Pedata F, Colotta V, Varano F, Coppi E, Pugliese AM. Venturini M, et al. Biomolecules. 2023 May 27;13(6):894. doi: 10.3390/biom13060894. Biomolecules. 2023. PMID: 37371474 Free PMC article.
References
- BONA E., ADEN U., GILLAND E., FREDHOLM B.B., HAGBERG H. Neonatal cerebral hypoxia-ischemia: the effect of adenosine receptor antagonists. Neuropharmacology. 1997;36:1327–1338. - PubMed
- CANHAO P., DE MENDONÇA A., RIBEIRO J.A. 1,3-Dipropyl-8-cyclopentylxanthine attenuates the NMDA response to hypoxia in the rat hippocampus. Brain Res. 1994;661:265–273. - PubMed
- CORRADETTI R., LO CONTE G., MORONI F., PASSANI M.B., PEPEU G. Adenosine decreases aspartate and glutamate release from rat hippocampal slices. Eur. J. Pharmacol. 1984;104:19–26. - PubMed
- CORRADETTI R., MONETI G., MORONI F., PEPEU G., WIERASZKO A. Electrical stimulation of the stratum radiatum increases the release and neosynthesis of aspartate, glutamate, and γ-aminobutyric acid in rat hippocampal slices. J. Neurochem. 1983;41:1518–1525. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous