The Pore Size of Non-Graminaceous Plant Cell Walls Is Rapidly Decreased by Borate Ester Cross-Linking of the Pectic Polysaccharide Rhamnogalacturonan II - PubMed (original) (raw)
The Pore Size of Non-Graminaceous Plant Cell Walls Is Rapidly Decreased by Borate Ester Cross-Linking of the Pectic Polysaccharide Rhamnogalacturonan II
A Fleischer et al. Plant Physiol. 1999 Nov.
Abstract
The walls of suspension-cultured Chenopodium album L. cells grown continually for more than 1 year on B-deficient medium contained monomeric rhamnogalacturonan II (mRG-II) but not the borate ester cross-linked RG II dimer (dRG-II-B). The walls of these cells had an increased size limit for dextran permeation, which is a measure of wall pore size. Adding boric acid to growing B-deficient cells resulted in B binding to the wall, the formation of dRG-II-B from mRG-II, and a reduction in wall pore size within 10 min. The wall pore size of denatured B-grown cells was increased by treatment at pH </= 2.0 or by treatment with Ca(2+)-chelating agents. The acid-mediated increase in wall pore size was prevented by boric acid alone at pH 2.0 and by boric acid together with Ca(2+), but not by Na(+) or Mg(2+) ions at pH 1.5. The Ca(2+)-chelator-mediated increase in pore size was partially reduced by boric acid. Our results suggest that B-mediated cross-linking of RG-II in the walls of living plant cells generates a pectin network with a decreased size exclusion limit for polymers. The formation, stability, and possible functions of a borate ester cross-linked pectic network in the primary walls of nongraminaceous plant cells are discussed.
Figures
Figure 1
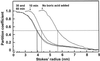
The B-dependent decrease in the MSL of growing B-deficient C. album cells. Boric acid (100 μ
m
) was added to a growing suspension of B-deficient C. album cells 2 d after their subcultivation. The cells were collected at the times shown and then denatured with ethanol. The denatured and rehydrated cells (from 1 g fresh weight) were equilibrated for 30 min with a polydisperse dextran probing solution (1 mL). The molecular size distribution of the dextrans was modified by their partial diffusion into the cell lumina. The modified dextran solution was fractionated by SEC and the eluate monitored with a polarimetric detector. The dextran partition curves were generated by computer analysis of the size-exclusion chromatograms (Dautzenberg et al., 1999). The curves show the dependence of the dextran partition coefficient on Stokes' radius of the dextran. The Stokes' radius obtained from each curve at a partition coefficient of 0.5 is designated as the MSL of the walls.
Figure 2
The decrease in wall pore size of growing B-deficient C. album cells and the binding of B to B-deficient cells after the addition of boric acid. A, Cells were grown continuously in the absence of added B. Boric acid (10 [▪] or 100 μ
m
[▴]) was added at time zero. No boric acid was added to control cells (♦). At the indicated times the cells were collected by centrifugation and denatured by treatment with ethanol. The MSL of the walls was determined by permeation of polydisperse dextrans into the lumen of the denatured cells. B, Cells were grown continuously in the absence of added B. One portion of the cells was saturated for 30 min with N2 gas (♦) and a second portion saturated with air (▪). Boric acid (100 μ
m
) was added and at the specified times the MSL of the walls was determined. C, Cells were grown continuously in the absence of added B. Boric acid (10 [▪] and 100 μ
m
[♦]) was added at time zero and at the indicated times, the cells were collected by centrifugation and washed with water to remove the soluble B. The wall-bound B was released by treatment with 1
n
sulfuric acid and chloroform-hexanediol and then quantified colorimetrically.
Figure 3
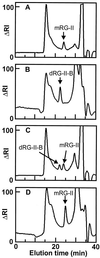
SEC with refractive index (RI) detection and SEC with ICP-MS detection of the saponified and polygalacturonase-treated phosphate buffer-soluble extracts of the AIR from C. album cells grown in the presence or absence of boric acid. A, SEC-RI profile of the extract from cells grown in the presence of boric acid (100 μ
m
). B, SEC-RI profile of the extract from cells grown in the absence of boric acid. C, SEC-RI profile of the extract from growing B-deficient cells that had been treated for 10 min with 10B boric acid (100 μ
m
). The RG-II dimer (dRG-II-B) and monomer (mRG-II) eluted at 21.9 and 23.8 min, respectively. The insets in A through C show the 11B and 10B profiles obtained by SEC-ICP-MS analysis of the extracts. The ICP-MS was operated in the selected ion mode to detect only the 11B and 10B isotopes. The peak at approximately 36 min corresponds to boric acid that originated from a contaminant in the eluant used for chromatography. The Superdex-75 column was calibrated with red wine dRG-II-B (approximately 9.4 kD) and red wine mRG-II (approximately 4.7 kD), which have retention times of 22.8 and 24.7 min, respectively. Dextrans of 40 and 25 kD eluted at 17 and 19.6 min, respectively. The _V_i of the column using Glc was 35 min.
Figure 4
Chemical interconversion of mRG-II and dRG-II-B from B-deficient and 10B-treated C. album cells. A, SEC-RI profile of the saponified and polygalacturonase-treated phosphate-buffer-soluble material from the AIR of C. album cells grown in the absence of added boric acid. B, SEC-RI profile of the extract shown in A after treatment for 24 h at pH 3.5 with boric acid (1 m
m
) and lead nitrate (1 m
m
). C, SEC-RI profile of the saponified and polygalacturonase-treated phosphate-buffer-soluble extract from the AIR of B-deficient C. album cells grown for 10 min in the presence of 10B boric acid (100 μ
m
). D, SEC-RI profile of the extract shown in C after treatment for 30 min at pH 1.0.
Similar articles
- Formation of rhamnogalacturonan II-borate dimer in pectin determines cell wall thickness of pumpkin tissue.
Ishii T, Matsunaga T, Hayashi N. Ishii T, et al. Plant Physiol. 2001 Aug;126(4):1698-705. doi: 10.1104/pp.126.4.1698. Plant Physiol. 2001. PMID: 11500567 Free PMC article. - Pectic polysaccharide rhamnogalacturonan II is covalently linked to homogalacturonan.
Ishii T, Matsunaga T. Ishii T, et al. Phytochemistry. 2001 Jul;57(6):969-74. doi: 10.1016/s0031-9422(01)00047-4. Phytochemistry. 2001. PMID: 11423143 - Rhamnogalacturonan II: structure and function of a borate cross-linked cell wall pectic polysaccharide.
O'Neill MA, Ishii T, Albersheim P, Darvill AG. O'Neill MA, et al. Annu Rev Plant Biol. 2004;55:109-39. doi: 10.1146/annurev.arplant.55.031903.141750. Annu Rev Plant Biol. 2004. PMID: 15377216 Review. - Arabinogalactan-Proteins as Boron-Acting Enzymes, Cross-Linking the Rhamnogalacturonan-II Domains of Pectin.
Begum RA, Fry SC. Begum RA, et al. Plants (Basel). 2023 Nov 21;12(23):3921. doi: 10.3390/plants12233921. Plants (Basel). 2023. PMID: 38068557 Free PMC article. Review.
Cited by
- Making and breaking of boron bridges in the pectic domain rhamnogalacturonan-II at apoplastic pH in vivo and in vitro.
Begum RA, Messenger DJ, Fry SC. Begum RA, et al. Plant J. 2023 Mar;113(6):1310-1329. doi: 10.1111/tpj.16112. Epub 2023 Feb 8. Plant J. 2023. PMID: 36658763 Free PMC article. - Germanium does not substitute for boron in cross-linking of rhamnogalacturonan II in pumpkin cell walls.
Ishii T, Matsunaga T, Iwai H, Satoh S, Taoshita J. Ishii T, et al. Plant Physiol. 2002 Dec;130(4):1967-73. doi: 10.1104/pp.009514. Plant Physiol. 2002. PMID: 12481079 Free PMC article. - The gene expression and enzyme activity of plant 3-deoxy-D-manno-2-octulosonic acid-8-phosphate synthase are preferentially associated with cell division in a cell cycle-dependent manner.
Delmas F, Petit J, Joubès J, Séveno M, Paccalet T, Hernould M, Lerouge P, Mouras A, Chevalier C. Delmas F, et al. Plant Physiol. 2003 Sep;133(1):348-60. doi: 10.1104/pp.103.026872. Plant Physiol. 2003. PMID: 12970500 Free PMC article. - Effects of fuzzless cottonseed phenotype on cottonseed nutrient composition in near isogenic cotton (Gossypium hirsutum L.) mutant lines under well-watered and water stress conditions.
Bellaloui N, Turley RB. Bellaloui N, et al. Front Plant Sci. 2013 Dec 30;4:516. doi: 10.3389/fpls.2013.00516. eCollection 2013. Front Plant Sci. 2013. PMID: 24416037 Free PMC article. - Boron Deficiency in Trifoliate Orange Induces Changes in Pectin Composition and Architecture of Components in Root Cell Walls.
Wu X, Riaz M, Yan L, Du C, Liu Y, Jiang C. Wu X, et al. Front Plant Sci. 2017 Nov 8;8:1882. doi: 10.3389/fpls.2017.01882. eCollection 2017. Front Plant Sci. 2017. PMID: 29167675 Free PMC article.
References
- Baron-Epel O, Gharyal PK, Schindler M. Pectins as mediators of wall porosity in soybean cells. Planta. 1988;175:389–395. - PubMed
- Behrendt U, Zoglauer K. Boron controls suspensor development in embryogenic cultures of Larix decidua. Physiol Plant. 1996;97:321–326.
- Blumenkrantz NJ, Asboe-Hansen B. A new method for the determination of uronic acid. Anal Biochem. 1973;54:484–489. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous