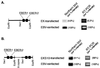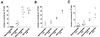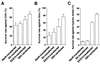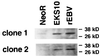Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata - PubMed (original) (raw)
Oncogenic role of Epstein-Barr virus-encoded RNAs in Burkitt's lymphoma cell line Akata
J Komano et al. J Virol. 1999 Dec.
Abstract
Our previous reports indicated that Epstein-Barr virus (EBV) contributes to the malignant phenotype and resistance to apoptosis in Burkitt's lymphoma (BL) cell line Akata (N. Shimizu, A. Tanabe-Tochikura, Y. Kuroiwa, and K. Takada, J. Virol. 68:6069-6073, 1994; J. Komano, M. Sugiura, and K. Takada, J. Virol. 72:9150-9156, 1998). Here we report that the EBV-encoded small RNAs (EBERs) are responsible for these phenotypes. Transfection of the EBER genes into EBV-negative Akata clones restored the capacity for growth in soft agar, tumorigenicity in SCID mice, resistance to apoptotic inducers, and upregulated expression of bcl-2 oncoprotein that were originally retained in parental EBV-positive Akata cells and lost in EBV-negative subclones. This is the first report which provides evidence that virus-encoded RNAs (EBERs) have oncogenic functions in BL cells.
Figures
FIG. 1
Relative EBER expression in EK- and EKS10-transfected Akata cells compared with EBV-reinfected Akata cells. For each EBER transfectant derived from different EBV-negative clones, more than 50 cell clones were examined for EBER expression, and 4 to 6 clones with the highest levels of EBER expression were chosen for further studies. Representative results are shown. Four EBER-transfected cell clones from an EBV-negative Akata cell clone were mixed and subjected to quantitative assays for EBER. (A) Schematic drawing of the _Eco_RI K fragment of Akata EBV DNA (left) and relative EBER expression in EK-transfected cells (right). (B) Schematic drawing of EKS10 (left) and relative EBER expression in EKS10-transfected cells (right). avr, average.
FIG. 2
Colony production in soft agar for _neoR_-transfected, EK-transfected, EKS10-transfected, and EBV-reinfected cells. The individual panels show independent experiments with different EBV-negative Akata cell clones. Each dot represents the number of visible colonies emerging per 104 cells. Horizontal bars represent the mean values for each group. The differences between _neoR_-transfected and EKS10-transfected cell clones were significant (P, <0.01), as determined by the Mann-Whitney U test.
FIG. 3
Histological examination of tumors derived from EKS10-transfected Akata cells in SCID mice. Adjacent sections from paraffin-embedded blocks were stained by hematoxylin-eosin or subjected to an in situ hybridization study. Typical lymphoma histology was observed (left panel; magnification, ×400). The EBER-1 in situ hybridization study revealed that almost all the tumor cells were positive for EBER-1 expression (middle panel; magnification, ×400). Dark signals representing the presence of EBER-1 transcripts were specifically present in the nuclei of tumor cells. A negative control for the EBER-1 in situ hybridization study is also shown (right panel; magnification, ×400).
FIG. 4
Resistance to apoptosis of _neoR_-transfected, EK-transfected, EKS10-transfected, and EBV-reinfected cells tested with CHX (A), glucocorticoid (Gluc) (B), and hypoxic stress (C). The bars show the means ± standard deviations for six clones. The data are typical results from three independent experiments. As determined by a t test analysis, the differences between mean values for _neoR_- and EKS10-transfected cells were significant at P values of <0.01 (CHX), <0.05 (Gluc), and <0.001 (hypoxic stress).
FIG. 5
Expression of bcl-2 protein in _neoR_-transfected, EKS10-transfected, and EBV-reinfected (rEBV) cells derived from two independent EBV-negative Akata cell clones. The data are typical results from several experiments.
Similar articles
- Epstein-Barr virus contributes to the malignant phenotype and to apoptosis resistance in Burkitt's lymphoma cell line Akata.
Komano J, Sugiura M, Takada K. Komano J, et al. J Virol. 1998 Nov;72(11):9150-6. doi: 10.1128/JVI.72.11.9150-9156.1998. J Virol. 1998. PMID: 9765461 Free PMC article. - Role of Epstein-Barr virus in Burkitt's lymphoma.
Takada K. Takada K. Curr Top Microbiol Immunol. 2001;258:141-51. doi: 10.1007/978-3-642-56515-1_9. Curr Top Microbiol Immunol. 2001. PMID: 11443859 Review. - Role of bcl-2 in Epstein-Barr virus-induced malignant conversion of Burkitt's lymphoma cell line Akata.
Komano J, Takada K. Komano J, et al. J Virol. 2001 Feb;75(3):1561-4. doi: 10.1128/JVI.75.3.1561-1564.2001. J Virol. 2001. PMID: 11152530 Free PMC article. - Epstein-Barr virus RNA confers resistance to interferon-alpha-induced apoptosis in Burkitt's lymphoma.
Nanbo A, Inoue K, Adachi-Takasawa K, Takada K. Nanbo A, et al. EMBO J. 2002 Mar 1;21(5):954-65. doi: 10.1093/emboj/21.5.954. EMBO J. 2002. PMID: 11867523 Free PMC article. - The role of Epstein-Barr virus-encoded small RNAs (EBERs) in oncogenesis.
Nanbo A, Takada K. Nanbo A, et al. Rev Med Virol. 2002 Sep-Oct;12(5):321-6. doi: 10.1002/rmv.363. Rev Med Virol. 2002. PMID: 12211044 Review.
Cited by
- From virus to cancer: Epstein-Barr virus miRNA connection in Burkitt's lymphoma.
Jalilian S, Bastani MN. Jalilian S, et al. Infect Agent Cancer. 2024 Oct 18;19(1):54. doi: 10.1186/s13027-024-00615-1. Infect Agent Cancer. 2024. PMID: 39425210 Free PMC article. Review. - Regulation mechanism of EBV-encoded EBER1 and LMP2A on YAP1 and the impact of YAP1 on the EBV infection status in EBV-associated gastric carcinoma.
Sun Y, Shi D, Sun J, Zhang Y, Liu W, Luo B. Sun Y, et al. Virus Res. 2024 May;343:199352. doi: 10.1016/j.virusres.2024.199352. Epub 2024 Mar 11. Virus Res. 2024. PMID: 38462175 Free PMC article. - Establishment of Epstein-Barr Virus (EBV) Latent Gene-Expressing T-Cell Lines with an Expression Vector Harboring EBV Nuclear Antigen 1.
Kanno H, Osada T, Tateishi A. Kanno H, et al. Microorganisms. 2023 Oct 25;11(11):2624. doi: 10.3390/microorganisms11112624. Microorganisms. 2023. PMID: 38004636 Free PMC article. - Epstein-Barr Virus History and Pathogenesis.
Yu H, Robertson ES. Yu H, et al. Viruses. 2023 Mar 9;15(3):714. doi: 10.3390/v15030714. Viruses. 2023. PMID: 36992423 Free PMC article. Review. - Shaping the host cell environment with viral noncoding RNAs.
Gorbea C, Elhakiem A, Cazalla D. Gorbea C, et al. Semin Cell Dev Biol. 2023 Sep 15;146:20-30. doi: 10.1016/j.semcdb.2022.12.008. Epub 2022 Dec 28. Semin Cell Dev Biol. 2023. PMID: 36581481 Free PMC article. Review.
References
- Askew D S, Ashmun R A, Simmons B C, Cleveland J L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991;6:1915–1922. - PubMed
- Bissonnette R P, Echeverri F, Mahboubi A, Green D R. Apoptotic cell death induced by c-myc is inhibited by bcl-2. Nature. 1992;359:552–554. - PubMed
- Brito-Babapulle V, Crawford A, Khokhar T, Laffan M, Matutes E, Fairhead S, Catovsky D. Translocations t(14;18) and t(8;14) with rearranged bcl-2 and c-myc in a case presenting as B-ALL (L3) Leukemia. 1991;5:83–87. - PubMed
- Chang K L, Chen Y Y, Shibata D, Weiss L M. Description of an in situ hybridization methodology for detection of Epstein-Barr virus RNA in paraffin-embedded tissues, with a survey of normal and neoplastic tissues. Diagn Mol Pathol. 1992;1:246–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases