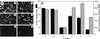Dynamin is required for recombinant adeno-associated virus type 2 infection - PubMed (original) (raw)
Dynamin is required for recombinant adeno-associated virus type 2 infection
D Duan et al. J Virol. 1999 Dec.
Abstract
Recombinant adeno-associated virus (rAAV) vectors for gene therapy of inherited disorders have demonstrated considerable potential for molecular medicine. Recent identification of the viral receptor and coreceptors for AAV type 2 (AAV-2) has begun to explain why certain organs may demonstrate higher efficiencies of gene transfer with this vector. However, the mechanisms by which AAV-2 enters cells remain unknown. In the present report, we have examined whether the endocytic pathways of rAAV-2 are dependent on dynamin, a GTPase protein involved in clathrin-mediated internalization of receptors and their ligands from the plasma membrane. Using a recombinant adenovirus expressing a dominant-inhibitory form of dynamin I (K44A), we have demonstrated that rAAV-2 infection is partially dependent on dynamin function. Overexpression of mutant dynamin I significantly inhibited AAV-2 internalization and gene delivery, but not viral binding. Furthermore, colocalization of rAAV and transferrin in the same endosomal compartment provides additional evidence that clathrin-coated pits are the predominant pathway for endocytosis of AAV-2 in HeLa cells.
Figures
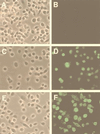
FIG. 1
Immunofluorescent detection of the K44A dominant-negative dynamin I mutant in HeLa cells following rAd-mediated expression. Fifty percent confluent HeLa cells were infected with rAd expressing β-galactosidase (A and B), HA-tagged IκB (C and D), or HA-tagged K44A dynamin I mutant (E and F) at an MOI of 1,000 particles/cell for 48 h in 2% FBS–DMEM. Cells were then fixed in 4% paraformaldehyde for 10 min and permeabilized in 0.2% Triton X-100 for 10 min. After blocking with 20% goat serum for 30 min, HA epitope was detected with a 1:100 dilution of mouse monoclonal anti-HA FITC-conjugated antibody (clone 12CA5; Boehringer Mannheim Corp.) Panels A, C, and E are Nomarski photomicrographs of the FITC fluorescent fields shown in panels B, D, and F, respectively.
FIG. 2
Expression of K44A dynamin I mutant inhibits rAAV-2-mediated gene transfer in HeLa cells. The effects of K44A dynamin I expression on the transduction efficiency of rAd (Ad.GFP) or rAAV (AV.GFP3ori) were evaluated. Eighty percent confluent HeLa cells were first infected with rAd expressing either K44A dynamin I or LacZ (MOI = 5,000 particles/cell) 48 h prior to infection with GFP-expressing rAd (MOI = 1,000 particles/cell) and rAAV (MOI = 1,000 particles/cell). Control experiments were also performed in which HeLa cells were infected with either Ad.GFP or AV.GFP3ori alone (both at MOI = 1,000 particles/cell). GFP transgene expression was detected at 24 h postinfection by either indirect fluorescent microscopy (A) or by flow cytometric analysis (B). Conditions in panels A and B are indicated numerically as follows: 1, infection with Ad.GFP alone; 2, preinfection with Ad.LacZ followed by superinfection with Ad.GFP; 3, preinfection with Ad.K44Adynamin followed by superinfection with Ad.GFP; 4, infection with AV.GFP3ori alone; 5, preinfection with Ad.LacZ followed by superinfection with AV.GFP3ori; 6, preinfection with Ad.K44Adynamin followed by superinfection with AV.GFP3ori. The data represent the mean ± standard error of three independent experiments.
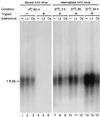
FIG. 3
Southern blot analysis of rAAV DNA entry into HeLa cells. HeLa cells were preinfected with either Ad.LacZ (Lz) or Ad.K44Adynamin (Dy) at an MOI of 5,000 particles/cell for 48 h. A control set of HeLa cells which were not preinfected with rAd (−) were also included in the study to assess for the baseline binding and internalization of rAAV in the absence of any modifications. The binding of rAAV to HeLa cells was determined by AV.GFP3ori infection (MOI = 1,000 particles/cell) at 4°C for 1 h followed by Hirt DNA analysis on Southern blots against 32P-labeled EGFP DNA probes (lanes 1, 2, and 3). Treatment of cells with trypsin prior to Hirt DNA extraction removed all cell-surface-bound rAAVs (lanes 4, 5, and 6). The extent of viral DNA endocytosis was determined by the fraction of trypsin-resistant internalized viral genome at 37°C for the various incubation times indicated. The 1.6-kb bands represent single-stranded rAAV DNA.
FIG. 4
Viral internalization, but not binding, is affected by overexpression of the K44A dynamin I mutant. HeLa cells (106) were preinfected with either Ad.LacZ or Ad.K44Adynamin (MOI = 5,000 particles/cell) for 48 h. Uninfected cells were also included as controls for the baseline binding and internalization in the absence of adenovirus preinfection. To quantify rAAV binding (A), HeLa cells were then infected with 9 × 104 cpm of 35S-labeled rAAV at 4°C for 1 h. After washing in ice-cold PBS three times, cells were lysed in 1× RIAP buffer (50 mM Tris 7.5, 150 mM NaCl, 1% Triton X-100, 1% Na-deoxycholate, 0.1% SDS), and radioactivity was quantified in a scintillation counter. Panel B depicts the net internalized 35S-labeled virus at 1, 6, and 12 h after rAAV infection. In this set of experiments, surface-bound virus was removed by trypsinization and PBS washing prior to cell lysis in 1× RIAP buffer and quantification of radioactivity. The data are the mean ± standard error of three independent samples.
FIG. 5
Colocalization of Cy3-labeled rAAV and fluorescein-labeled transferrin. To directly evaluate endocytosis of rAAV, 4 × 103 HeLa cells grown on glass slides were precooled at 4°C for 10 min and subsequently infected with 4 × 108 particles of Cy3-labeled rAAV at 4°C for 1 h (MOI = 100,000) in the presence of 15-μg/ml FITC-transferrin. Endocytosis of rAAV and FITC-transferrin was initiated by shifting cells to 37°C for 10 min. After extensive washing in ice-cold PBS, cells were fixed in 4% paraformaldehyde and mounted with Citifluor antifadent. Confocal fluorescence photomicrographs were taken for rAAV (A), transferrin (B), and combined FITC-rhodamine (C) channels. Arrows mark the colocalization of Cy3-rAAV and transferrin within the same endosome compartment.
Similar articles
- Induction of mutant dynamin specifically blocks endocytic coated vesicle formation.
Damke H, Baba T, Warnock DE, Schmid SL. Damke H, et al. J Cell Biol. 1994 Nov;127(4):915-34. doi: 10.1083/jcb.127.4.915. J Cell Biol. 1994. PMID: 7962076 Free PMC article. - Receptor-mediated Moloney murine leukemia virus entry can occur independently of the clathrin-coated-pit-mediated endocytic pathway.
Lee S, Zhao Y, Anderson WF. Lee S, et al. J Virol. 1999 Jul;73(7):5994-6005. doi: 10.1128/JVI.73.7.5994-6005.1999. J Virol. 1999. PMID: 10364351 Free PMC article. - Infectious entry pathway of adeno-associated virus and adeno-associated virus vectors.
Bartlett JS, Wilcher R, Samulski RJ. Bartlett JS, et al. J Virol. 2000 Mar;74(6):2777-85. doi: 10.1128/jvi.74.6.2777-2785.2000. J Virol. 2000. PMID: 10684294 Free PMC article. - Participation of dynamin in the biogenesis of cytoplasmic vesicles.
Henley JR, Cao H, McNiven MA. Henley JR, et al. FASEB J. 1999 Dec;13 Suppl 2:S243-7. doi: 10.1096/fasebj.13.9002.s243. FASEB J. 1999. PMID: 10619136 Review. - Multiple pathways for the dynamin-regulated internalization of muscarinic acetylcholine receptors.
van Koppen CJ. van Koppen CJ. Biochem Soc Trans. 2001 Aug;29(Pt 4):505-8. doi: 10.1042/bst0290505. Biochem Soc Trans. 2001. PMID: 11498018 Review.
Cited by
- A review of therapeutic prospects of non-viral gene therapy in the retinal pigment epithelium.
Koirala A, Conley SM, Naash MI. Koirala A, et al. Biomaterials. 2013 Sep;34(29):7158-67. doi: 10.1016/j.biomaterials.2013.06.002. Epub 2013 Jun 22. Biomaterials. 2013. PMID: 23796578 Free PMC article. Review. - Human rhinovirus type 2 is internalized by clathrin-mediated endocytosis.
Snyers L, Zwickl H, Blaas D. Snyers L, et al. J Virol. 2003 May;77(9):5360-9. doi: 10.1128/jvi.77.9.5360-5369.2003. J Virol. 2003. PMID: 12692238 Free PMC article. - Identification of amino acid residues in the capsid proteins of adeno-associated virus type 2 that contribute to heparan sulfate proteoglycan binding.
Opie SR, Warrington KH Jr, Agbandje-McKenna M, Zolotukhin S, Muzyczka N. Opie SR, et al. J Virol. 2003 Jun;77(12):6995-7006. doi: 10.1128/jvi.77.12.6995-7006.2003. J Virol. 2003. PMID: 12768018 Free PMC article. - Endosomal NADPH oxidase regulates c-Src activation following hypoxia/reoxygenation injury.
Li Q, Zhang Y, Marden JJ, Banfi B, Engelhardt JF. Li Q, et al. Biochem J. 2008 May 1;411(3):531-41. doi: 10.1042/BJ20071534. Biochem J. 2008. PMID: 18397177 Free PMC article. - Enhancement of adeno-associated virus infection by mobilizing capsids into and out of the nucleolus.
Johnson JS, Samulski RJ. Johnson JS, et al. J Virol. 2009 Mar;83(6):2632-44. doi: 10.1128/JVI.02309-08. Epub 2008 Dec 24. J Virol. 2009. PMID: 19109385 Free PMC article.
References
- Bennett J, Duan D, Engelhardt J F, Maguire A M. Real-time, noninvasive in vivo assessment of adeno-associated virus-mediated retinal transduction. Investig Ophthalmol Vis Sci. 1997;38:2857–2863. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources