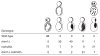The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast - PubMed (original) (raw)
The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast
W Bleazard et al. Nat Cell Biol. 1999 Sep.
Abstract
The dynamin-related GTPase Dnm1 controls mitochondrial morphology in yeast. Here we show that dnm1 mutations convert the mitochondrial compartment into a planar 'net' of interconnected tubules. We propose that this net morphology results from a defect in mitochondrial fission. Immunogold labelling localizes Dnm1 to the cytoplasmic face of constricted mitochondrial tubules that appear to be dividing and to the ends of mitochondrial tubules that appear to have recently completed division. The activity of Dnm1 is epistatic to that of Fzo1, a GTPase in the outer mitochondrial membrane that regulates mitochondrial fusion. dnm1 mutations prevent mitochondrial fragmentation in fzo1 mutant strains. These findings indicate that Dnm1 regulates mitochondrial fission, assembling on the cytoplasmic face of mitochondrial tubules at sites at which division will occur.
Figures
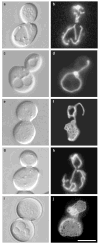
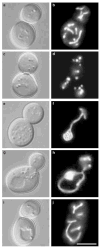
Figure 1. Mitochondrial membranes form nets in dnm1 mutant cells
Morphology of GFP-labelled mitochondrial membranes (pDO12) in b, wild-type (strain JSY3096), d, f, _dnm1_Δ (JSY3097), h, _mdm20_Δ (JSY3094), and j, _dnm1_Δ _mdm20_Δ (JSY3095) cells at 25 °C. The corresponding differential interference contrast images are shown in a, c, e, g, i. The four strains are sister spores from a single tetrad. Scale bar represents 5 μm.
Figure 2
Mitochondrial-membrane morphology in _dnm1_Δ and _dnm1_Δ _mdm20_Δ mutant cells.
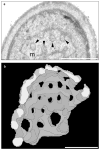
Figure 3. Transmission electron microscopy and serial reconstruction of a mitochondrial net
a, Section through a mitochondrial net (m) in a _dnm1_Δ _mdm20_Δ cell (strain JSY3095). Black arrowheads mark ‘holes’ in the mitochondrial compartment. b, Computer reconstruction of a mitochondrial net (grey and white) generated from 12 serial sections, including the image shown in a. Scale bars represent 1 μm.
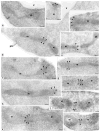
Figure 4. Dnm1 localizes to mitochondrial constriction sites and mitochondrial tips
The distribution of the Dnm1–HAcp protein (black arrowheads; strain JSY1781) was determined by immunogold labelling of ultrathin cryosections. Gold particles were found concentrated at the tips of mitochondrial tubules proximal to the plasma membrane (b, c, f), at the tips of mitochondrial tubules that may be newly divided (d, e, h), and at putative mitochondrial constriction/division sites (i–l). g, Gold particles are also detected on mitochondria at sites that are not constricted. a, No mitochondrial labelling was detected in cells lacking the Dnm1–HAcp protein (strain JSY3097). m, mitochondria; pm, plasma membrane; v, vacuole. Scale bars represent 0.1 μm.
Figure 5. dnm1 mutations block mitochondrial fragmentation in fzo1-1 cells
Morphology of GFP-labelled mitochondrial membranes (carrying plasmid pVT100UGFP) in b, wild-type (strain JSY3162), d, fzo1-1 (JSY3161), f, _dnm1_Δ (JSY3164), and h, j, _dnm1_Δ fzo1-1 (JSY3163) cells at 37 °C. The corresponding differential interference contrast images are shown in a, c, e, g, i. The four strains are sister spores from a single tetrad. Scale bar represents 5 μm.
Figure 6. Mitochondrial-membrane morphology in dnm1 and dnm1 fzo1-1 mutant cells
The asterisk indicates that 5% of _dnm1_Δ fzo1-1 cells contain visible nets at 37 °C.
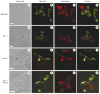
Figure 7. Mitochondrial fusion is not restored in dnm1 fzo1-1 cells
Wild-type (a–d), fzo1-1 (e–h), _dnm1_Δ (i–l) and _dnm1_Δ fzo1-1 (m–p) cells of opposite mating type were labelled with either mito-GFP (b, f, j, n) or MitoTracker CMXRos (c, g, k, o) and mated at 37 °C. Images of zygotes were obtained by confocal microscopy and mitochondrial fusion was assessed by examining the overlayed mito-GFP and MitoTracker images (d, h, l, p). The ‘z’ in i designates the zygote visualized in j–l. Scale bars represent 2 μm.
Similar articles
- Division versus fusion: Dnm1p and Fzo1p antagonistically regulate mitochondrial shape.
Sesaki H, Jensen RE. Sesaki H, et al. J Cell Biol. 1999 Nov 15;147(4):699-706. doi: 10.1083/jcb.147.4.699. J Cell Biol. 1999. PMID: 10562274 Free PMC article. - The intramitochondrial dynamin-related GTPase, Mgm1p, is a component of a protein complex that mediates mitochondrial fusion.
Wong ED, Wagner JA, Scott SV, Okreglak V, Holewinske TJ, Cassidy-Stone A, Nunnari J. Wong ED, et al. J Cell Biol. 2003 Feb 3;160(3):303-11. doi: 10.1083/jcb.200209015. J Cell Biol. 2003. PMID: 12566426 Free PMC article. - The dynamin-related GTPase, Mgm1p, is an intermembrane space protein required for maintenance of fusion competent mitochondria.
Wong ED, Wagner JA, Gorsich SW, McCaffery JM, Shaw JM, Nunnari J. Wong ED, et al. J Cell Biol. 2000 Oct 16;151(2):341-52. doi: 10.1083/jcb.151.2.341. J Cell Biol. 2000. PMID: 11038181 Free PMC article. - Mitochondrial dynamics in yeast.
Hermann GJ, Shaw JM. Hermann GJ, et al. Annu Rev Cell Dev Biol. 1998;14:265-303. doi: 10.1146/annurev.cellbio.14.1.265. Annu Rev Cell Dev Biol. 1998. PMID: 9891785 Review. - Fusion, fragmentation, and fission of mitochondria.
Polyakov VY, Soukhomlinova MY, Fais D. Polyakov VY, et al. Biochemistry (Mosc). 2003 Aug;68(8):838-49. doi: 10.1023/a:1025738712958. Biochemistry (Mosc). 2003. PMID: 12948383 Review.
Cited by
- Molecular mechanisms of mitochondrial dynamics.
Tábara LC, Segawa M, Prudent J. Tábara LC, et al. Nat Rev Mol Cell Biol. 2024 Oct 17. doi: 10.1038/s41580-024-00785-1. Online ahead of print. Nat Rev Mol Cell Biol. 2024. PMID: 39420231 Review. - Ribosomes hibernate on mitochondria during cellular stress.
Gemin O, Gluc M, Rosa H, Purdy M, Niemann M, Peskova Y, Mattei S, Jomaa A. Gemin O, et al. Nat Commun. 2024 Oct 8;15(1):8666. doi: 10.1038/s41467-024-52911-4. Nat Commun. 2024. PMID: 39379376 Free PMC article. - Dynamins combine mechano-constriction and membrane remodeling to enable two-step mitochondrial fission via a 'snap-through' instability.
Alimohamadi H, Luo EW, Yang R, Gupta S, Nolden KA, Mandal T, Blake Hill R, Wong GCL. Alimohamadi H, et al. bioRxiv [Preprint]. 2024 Aug 20:2024.08.19.608723. doi: 10.1101/2024.08.19.608723. bioRxiv. 2024. PMID: 39229060 Free PMC article. Preprint. - Beyond fission and fusion-Diving into the mysteries of mitochondrial shape.
Preminger N, Schuldiner M. Preminger N, et al. PLoS Biol. 2024 Jul 1;22(7):e3002671. doi: 10.1371/journal.pbio.3002671. eCollection 2024 Jul. PLoS Biol. 2024. PMID: 38949997 Free PMC article. Review. - Atg44/Mdi1/mitofissin facilitates Dnm1-mediated mitochondrial fission.
Furukawa K, Hayatsu M, Okuyama K, Fukuda T, Yamashita SI, Inoue K, Shibata S, Kanki T. Furukawa K, et al. Autophagy. 2024 Oct;20(10):2314-2322. doi: 10.1080/15548627.2024.2360345. Epub 2024 Jun 4. Autophagy. 2024. PMID: 38818923 Free PMC article.
References
- Williamson DH, et al. In: Genetics, Biogenesis and Bioenergetics of Mitochondria. Bandlow W, Schweyen RJ, Thomas DY, Wolf K, Kaudewitz F, editors. Walter de Gruyter; Berlin: 1976. pp. 99–115.
- Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–1312. - PubMed
- Tourte M, Mignotte F, Mounolou JC. Organization and replicative activity of the mitochondria of oogenic and previtellogenic oocytes in Xenopus laevis. Dev Growth Differ. 1981;23:9–21. - PubMed
- Fuller MT. In: The Development of Drosophila melanogaster. Bate M, Martinez-Arias A, editors. Cold Spring Harb. Lab. Press; Cold Spring Harbor: 1993. pp. 71–147.
- Bereiter-Hahn J. Behavior of mitochondria in the living cell. Int Rev Cytol. 1990;122:1–63. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases