Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration - PubMed (original) (raw)
Skeletal myogenic progenitors originating from embryonic dorsal aorta coexpress endothelial and myogenic markers and contribute to postnatal muscle growth and regeneration
L De Angelis et al. J Cell Biol. 1999.
Abstract
Skeletal muscle in vertebrates is derived from somites, epithelial structures of the paraxial mesoderm, yet many unrelated reports describe the occasional appearance of myogenic cells from tissues of nonsomite origin, suggesting either transdifferentiation or the persistence of a multipotent progenitor. Here, we show that clonable skeletal myogenic cells are present in the embryonic dorsal aorta of mouse embryos. This finding is based on a detailed clonal analysis of different tissue anlagen at various developmental stages. In vitro, these myogenic cells show the same morphology as satellite cells derived from adult skeletal muscle, and express a number of myogenic and endothelial markers. Surprisingly, the latter are also expressed by adult satellite cells. Furthermore, it is possible to clone myogenic cells from limbs of mutant c-Met-/- embryos, which lack appendicular muscles, but have a normal vascular system. Upon transplantation, aorta-derived myogenic cells participate in postnatal muscle growth and regeneration, and fuse with resident satellite cells.The potential of the vascular system to generate skeletal muscle cells may explain observations of nonsomite skeletal myogenesis and raises the possibility that a subset of satellite cells may derive from the vascular system.
Figures
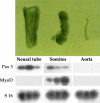
Figure 1
Top, Morphology of embryonic structures isolated from E9.5 mouse embryos after pancreatin digestion. Bottom, RT-PCR revealed the medial markers MyoD and Pax3 were expressed in dissected somites (different ratio of Pax3 to MyoD in different lanes depends upon isolation of somites at different cranio–caudal level); Pax3, but not MyoD, in the neural tube; and none of the markers was detectable in dissected dorsal aorta.
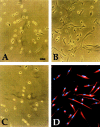
Figure 2
Morphology of typical clones derived from explant cultures of E9.5 limb bud (A), somites (B), and dorsal aorta (C), cultured in growth medium. Immunofluorescence analysis with antisarcomeric myosin antibody of a clone from dorsal aorta, after three days of culture in differentiation medium is shown in D. Bar, 15 μm.
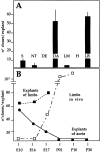
Figure 3
A, Quantitative analysis of satellite cell-like clones (shown in Fig. 1) from explant cultures of different anlagen of E9.5 embryos. Each bar is the average of at least three separate experiments, each performed in triplicate. B, Time course of the appearance of satellite cell-like clones from explants of vessels (___) or limb buds (- - -) at successive periods of development. The time course of the appearance of satellite cell-like clones, directly cloned from limb buds in vivo is also shown (– · –).
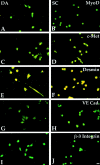
Figure 4
Myogenic and endothelial markers are expressed in aorta-derived myogenic cells and in satellite cells. Immunofluorescence analysis with antibodies against MyoD (A and B), c-Met (C and D), Desmin (E and F), VE-cadherin (G and H), and β-3 integrin (I and J) of clones derived from E9.5 dorsal aorta (A, C, E, G, and I) or P10 satellite cells (B, D, F, H, and J). Bar, 10 μm.
Figure 5
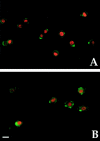
MyoD and VE-cadherin are coexpressed in aorta-derived myogenic cells and in satellite cells. Double immunofluorescence analysis with antibodies against MyoD (red) and VE-cadherin (green) of clones derived from E9.5 dorsal aorta (A) and of adult satellite cells (B). Bar, 10 μm.
Figure 6
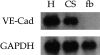
The message for VE-cadherin is expressed in adult satellite cells. Northern blot analysis of VE-cadherin expression in E 9.5 embryonic hearts (H), adult satellite cells (CS), and primary fibroblasts (fb).
Figure 7
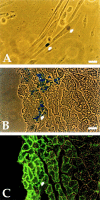
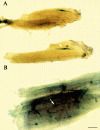
Myogenic clones are present in the limbs of c-Met–deficient embryos. Phase-contrast microscopy of wt (A) and MetD (B) E14 embryos showing normal vasculature in mutant embryos (arrow). β-galactosidase staining of wt (C) and MetD (D) crossed to MLC3F-nLacZ embryos showing complete absence of muscle (β-gal+) cells in the limb of E15 mutant embryos. Immunofluorescence analysis with antibodies against MyoD of clones derived from E13 limb buds of wt (E) or MetD (F and G) embryos. Nuclear staining (Hoechst) is shown in H, J, and K. Bar, 10 μm.
Figure 8
Aorta-derived myogenic cells undergo myogenesis in vitro and in vivo. A, Cocultures of clones from E9.5 dorsal aorta from MLC3F-nLacZ embryos and P10 wt satellite cells. Arrows indicate one β-gal+ and one β-gal− nucleus within the same myotube. Bar, 10 μm. B, Cross-section of a regenerating TA of a SCID/bg injected with pooled clones of satellite cell-like clones, showing a cluster of β-gal+ nuclei (arrow) within small regenerating fibers, labeled with an antibody against laminin in C. Bar, 25 μm.
Figure 9
Host-derived myogenic cells are present in fetal limbs transplanted under the skin of newborn MLC3F-nLacZ transgenic mice and vascularized by the host. A, Whole-mount stain reveals a cluster of β-gal+ nuclei in the transplanted limb. B–D, Cross-section of the same sample stained for β-galactosidase activity revealed the β-gal+ nuclei (B) inside myosin positive muscle fibers (D) adjacent to VE-cadherin positive vessels (C). Bar, 25 μm.
Figure 10
Aorta-derived myogenic cells contribute to growing fibers and also circulate. A, Whole-mount stain reveals clusters of β-gal+ nuclei (arrow) in the TA of P14 SCID/bg mice, two weeks after transplantation of embryonic aortas from E9 MLC3F-nLacZ embryos. B, Whole-mount stain of the contralateral TA of the same SCID/bg reveals several β-gal+ nuclei (arrow) dispersed throughout the whole muscle. Bar, 50 μm.
Comment in
- Myogenic shape-shifters.
Ordahl CP. Ordahl CP. J Cell Biol. 1999 Nov 15;147(4):695-8. doi: 10.1083/jcb.147.4.695. J Cell Biol. 1999. PMID: 10562273 Free PMC article. Review. No abstract available.
Similar articles
- Skeletal myogenic progenitors in the endothelium of lung and yolk sac.
Cusella De Angelis MG, Balconi G, Bernasconi S, Zanetta L, Boratto R, Galli D, Dejana E, Cossu G. Cusella De Angelis MG, et al. Exp Cell Res. 2003 Nov 1;290(2):207-16. doi: 10.1016/s0014-4827(03)00314-8. Exp Cell Res. 2003. PMID: 14567980 - Activation of different myogenic pathways: myf-5 is induced by the neural tube and MyoD by the dorsal ectoderm in mouse paraxial mesoderm.
Cossu G, Kelly R, Tajbakhsh S, Di Donna S, Vivarelli E, Buckingham M. Cossu G, et al. Development. 1996 Feb;122(2):429-37. doi: 10.1242/dev.122.2.429. Development. 1996. PMID: 8625794 - [Early stages of myogenesis as seen through the action of the myf-5 gene].
Buckingham M. Buckingham M. C R Seances Soc Biol Fil. 1997;191(1):43-54. C R Seances Soc Biol Fil. 1997. PMID: 9181127 French. - [Notch pathway: from development to regeneration of skeletal muscle].
Mayeuf A, Relaix F. Mayeuf A, et al. Med Sci (Paris). 2011 May;27(5):521-6. doi: 10.1051/medsci/2011275018. Epub 2011 May 25. Med Sci (Paris). 2011. PMID: 21609674 Review. French. - The formation of skeletal muscle: from somite to limb.
Buckingham M, Bajard L, Chang T, Daubas P, Hadchouel J, Meilhac S, Montarras D, Rocancourt D, Relaix F. Buckingham M, et al. J Anat. 2003 Jan;202(1):59-68. doi: 10.1046/j.1469-7580.2003.00139.x. J Anat. 2003. PMID: 12587921 Free PMC article. Review.
Cited by
- Endothelial cell signature in muscle stem cells validated by VEGFA-FLT1-AKT1 axis promoting survival of muscle stem cell.
Verma M, Asakura Y, Wang X, Zhou K, Ünverdi M, Kann AP, Krauss RS, Asakura A. Verma M, et al. Elife. 2024 Jun 6;13:e73592. doi: 10.7554/eLife.73592. Elife. 2024. PMID: 38842166 Free PMC article. - Single-Cell RNA-Sequencing Provides Insight into Skeletal Muscle Evolution during the Selection of Muscle Characteristics.
Xu D, Wan B, Qiu K, Wang Y, Zhang X, Jiao N, Yan E, Wu J, Yu R, Gao S, Du M, Liu C, Li M, Fan G, Yin J. Xu D, et al. Adv Sci (Weinh). 2023 Dec;10(35):e2305080. doi: 10.1002/advs.202305080. Epub 2023 Oct 23. Adv Sci (Weinh). 2023. PMID: 37870215 Free PMC article. - Semaphorins: Missing Signals in Age-dependent Alteration of Neuromuscular Junctions and Skeletal Muscle Regeneration.
Fard D, Barbiera A, Dobrowolny G, Tamagnone L, Scicchitano BM. Fard D, et al. Aging Dis. 2024 Apr 1;15(2):517-534. doi: 10.14336/AD.2023.0801. Aging Dis. 2024. PMID: 37728580 Free PMC article. Review. - The Role of Supporting Cell Populations in Satellite Cell Mediated Muscle Repair.
Johnson AL, Kamal M, Parise G. Johnson AL, et al. Cells. 2023 Jul 30;12(15):1968. doi: 10.3390/cells12151968. Cells. 2023. PMID: 37566047 Free PMC article. Review. - Mesoangioblasts at 20: From the embryonic aorta to the patient bed.
Cossu G, Tonlorenzi R, Brunelli S, Sampaolesi M, Messina G, Azzoni E, Benedetti S, Biressi S, Bonfanti C, Bragg L, Camps J, Cappellari O, Cassano M, Ciceri F, Coletta M, Covarello D, Crippa S, Cusella-De Angelis MG, De Angelis L, Dellavalle A, Diaz-Manera J, Galli D, Galli F, Gargioli C, Gerli MFM, Giacomazzi G, Galvez BG, Hoshiya H, Guttinger M, Innocenzi A, Minasi MG, Perani L, Previtali SC, Quattrocelli M, Ragazzi M, Roostalu U, Rossi G, Scardigli R, Sirabella D, Tedesco FS, Torrente Y, Ugarte G. Cossu G, et al. Front Genet. 2023 Jan 4;13:1056114. doi: 10.3389/fgene.2022.1056114. eCollection 2022. Front Genet. 2023. PMID: 36685855 Free PMC article. Review.
References
- Armand O., Boutineau A.M., Mauger A., Pautou M.P., Kieny M. Origin of satellite cells in avian skeletal muscles. Arch. Anat. Microsc. Morphol. Exp. 1983;72:163–181 . - PubMed
- Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967 . - PubMed
- Bianco P., Cossu G. Uno, nessuno e centomilasearching for the identity of mesodermal progenitors. Exp. Cell Res. 1999;251:257–263 . - PubMed
- Bischoff R. The satellite cell and muscle regeneration. In: Engel A.G., Franzini-Armstrong C., editors. Myology. 2nd ed. McGraw-Hill; NY : 1994. pp. 97–133.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous