Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport - PubMed (original) (raw)
Cytoplasmic dynein, the dynactin complex, and kinesin are interdependent and essential for fast axonal transport
M Martin et al. Mol Biol Cell. 1999 Nov.
Free PMC article
Abstract
In axons, organelles move away from (anterograde) and toward (retrograde) the cell body along microtubules. Previous studies have provided compelling evidence that conventional kinesin is a major motor for anterograde fast axonal transport. It is reasonable to expect that cytoplasmic dynein is a fast retrograde motor, but relatively few tests of dynein function have been reported with neurons of intact organisms. In extruded axoplasm, antibody disruption of kinesin or the dynactin complex (a dynein activator) inhibits both retrograde and anterograde transport. We have tested the functions of the cytoplasmic dynein heavy chain (cDhc64C) and the p150(Glued) (Glued) component of the dynactin complex with the use of genetic techniques in Drosophila. cDhc64C and Glued mutations disrupt fast organelle transport in both directions. The mutant phenotypes, larval posterior paralysis and axonal swellings filled with retrograde and anterograde cargoes, were similar to those caused by kinesin mutations. Why do specific disruptions of unidirectional motor systems cause bidirectional defects? Direct protein interactions of kinesin with dynein heavy chain and p150(Glued) were not detected. However, strong dominant genetic interactions between kinesin, dynein, and dynactin complex mutations in axonal transport were observed. The genetic interactions between kinesin and either Glued or cDhc64C mutations were stronger than those between Glued and cDhc64C mutations themselves. The shared bidirectional disruption phenotypes and the dominant genetic interactions demonstrate that cytoplasmic dynein, the dynactin complex, and conventional kinesin are interdependent in fast axonal transport.
Figures
Figure 1
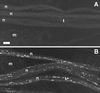
Mutations in cDhc64C cause posterior paralysis and defects in fast axonal transport. (A and B) Single video frames of third instar larvae (∼3 mm in length) crawling forward (to the left). (A) The control, heterozygous for a severe cDhc64C mutation (cDhc64C ek1 /+), exhibited wild-type posture. (B) The mutant, carrying a moderate mutation over a deletion [cDhc64C 6–10 _/Df(3L)10H_], exhibited posterior paralysis whereby the distal-most segments curved upward. (C–F) Laser scanning confocal micrographs of control, P{cDhc64C + }/+; cDhc64C 6–10 /Df(3L)10H (C and E), and mutant, cDhc64C 6–10 /Df(3L)10H (D and F), larval segmental nerves stained for tubulin (C and D) and synaptotagmin (E and F). Segmental nerves contain motor and sensory axons that connect the ventral ganglia to the body wall muscles and sensory organs in the abdominal segments. These fields of view each cover about half an abdominal segment. (C and D) Microtubules run longitudinally in the nerves and appeared unaffected by the cDhc64C mutations. A predominantly longitudinal and striated staining pattern was observed in both control and mutant nerves. The meshwork of single microtubules in the background was in muscle cells underlying the nerves. (E) Synaptotagmin staining was distributed in a punctate pattern along the nerve, presumably representing the distribution of anterograde fast transport vesicles. (F) Synaptotagmin in the mutant nerve was much more abundant and concentrated in large accumulations. n, segmental nerve; m, body-wall muscle. Bars, 10 μm.
Figure 2
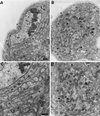
Mutations in cDhc64C cause axonal swellings filled with fast retrograde and anterograde organelles. Transmission electron micrographs of heterozygous control, Df(3L)10H/+ (A and C), and mutant, cDhc64C 6–10 /Df(3L)10H (B and D), larval segmental nerve cross-sections. (A) One-fourth of a large nerve containing a central cluster of axons (arrows) supported by glial cells (g) and a layer of extracellular matrix (e) is shown. A peripheral glial cell nucleus is also in view (gn). (C) A view of the same section at higher magnification; the predominant axonal organelles were mitochondria and small vesicles. The two axons from A are indicated by triplets of arrows. (B) The overall organization of the mutant nerve and its axons was normal (same magnification as in A). However, some axons were swollen (arrowheads). (D) This higher-magnification view of the largest swollen axon from B (same magnification as in C) contained many prelysosomal bodies (p), multivesicular bodies (b), mitochondria (m), and vesicles. Normal-sized axons are indicated by arrows. Bars, 500 nm.
Figure 3
Dominant negative mutation of Glued causes defects in fast axonal transport. Laser scanning confocal micrographs of larval segmental nerves from animals homozygous for Phs-Gl t stained for synaptotagmin are shown. (A) A control animal expressing wild-type p150Glued exhibited normal synaptotagmin staining. (B) A test animal expressing a dominant negative form of p150Glued exhibited accumulations of synaptotagmin that denote axonal swellings that contain fast anterograde cargoes. n, segmental nerve; t, tracheal tube; m, muscle. Bar, 10 μm.
Figure 4
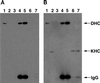
Immunoprecipitation of cDHC does not coprecipitate KHC. Drosophila cytosol fractions were separated by gel electrophoresis and transferred to a membrane. The membrane was stained first for cDHC (A) and subsequently for KHC (B). Thus, the cDHC bands are present in both panels. The experiment shown here was conducted with ovary cytosol from DHC-3HA–expressing flies immunoprecipitated with anti-HA mAb–agarose bead complexes. However, comparable results were obtained with DHC-3HA head extracts and either ovary or head wild-type extracts with the use of DHC mAbs for the immunoprecipitation. (Lanes 1) Proteins that pelleted from cytosol with taxol-stabilized microtubules in the absence of ATP. cDHC but not KHC was efficiently bound to microtubules under these conditions. (Lanes 2) KHC but not DHC was readily detected in cytosol. (Lanes 3) The pellet from cytosol that was incubated with unconjugated agarose beads. (Lanes 4 and 5) The pellets from cytosol that was incubated with beads conjugated with antibodies to cDHC via the 3HA tag; incubation was at either 4°C (lanes 4) or 25°C (lanes 5). (Lanes 6 and 7) The supernatants of the pellets shown in lanes 4 and 5, respectively. KHC remained in the immunoprecipitation supernatants, and cDHC was detected in the pellets. IgG, immunoglobulin G.
Figure 5
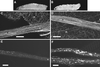
Mutations in Khc interact with mutations in Klc, cDhc64C, and Gl to produce the posterior-paralysis phenotype. Single video frames of wandering third instar larvae crawling forward (toward the left) are shown. All of the animals were heterozygous for a Khc null allele (Khc 16 /+). The animals in B, C, and D were also heterozygous for severe mutations in Klc [_Df(3L)34ex5/+_], cDhc64C (cDhc64C ek1 /+), and Gl (Gl 1 /+), respectively. The larva in A showed normal locomotion, whereas the double heterozygous larvae all showed a synthetic posterior-paralysis phenotype, as indicated by the upturned tails.
Figure 6
Mutations in Khc interact with mutations in Klc, cDhc64C, and Gl to produce axonal swellings. Laser scanning confocal micrographs of segmental nerves from third instar larvae stained for synaptotagmin are shown. The genotypes are as follows: (A) wild type; (B) Khc 6 /+ (hypomorphic allele); (C) Khc 16 /+ (null allele); (D) Df(3L)34ex5/+ (Klc deletion); (E) Khc 16 /+; Df(3L)34ex5/+; (F) Khc 16 /+; Df(3L)34ex5, +/+, P{Khc+}; (G) cDhc64C_ek1_ /+; (H) Khc16/+; cDhc64Cek1/+; (I) Khc16/+; cDhc64Cek1, +/+, P{Khc+}; (J) Gl_1_ /+; (K) Khc16/+; Gl1/+; (L) Khc16/+; Gl1, +/+, P{Khc+}; (M) Df(3L)10H/+ (cDhc64C deletion); (N) Df(3L)10H, +/+, Gl1; and (O) cDhc64Cek1, +/+, Gl 1. (A, B, G, J, and M) Axonal swellings were rarely seen in wild type, heterozygous hypomorphic Khc mutations, or animals heterozygous for severe cDhc64C or Gl alleles. (C and D) Heterozygotes for severe Khc and Klc mutations did have axon swellings in some regions but showed no swellings in other regions. (E, H, and K) Combination of Klc, cDhc64C, or Gl mutations with Khc in double heterozygotes caused dramatic increases in the number of axon swellings relative to the respective single heterozygous animals (compare C and D with E; compare C and G with H; compare C and J with K). In contrast, combination of the most severe alleles of cDhc64C and Gl led to only a slight increase in axon swellings (compare J and M with N and then compare G and M with O). (F, I, and L) The axon swellings caused by double heterozygous combinations with Khc null mutations were rescued by a single transgenic copy of wild-type Khc. Bar, 10 μm. b, boutons of an underlying neuromuscular junction; n, segmental nerve; t, tracheal tube. All micrographs are at the same magnification.
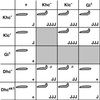
Figure 7
Summary of the dominant genetic interactions observed between Khc, Klc, cDhc64C, and Gl. The gene symbols at the top and left represent heterozygous mutant contributions. The internal rectangles contain information on the double heterozygous combinations indicated by the respective row and column. Khc−, Klc−, and Dhc− represent multiple severe alleles and deletions tested for each of these genes. In each box, the upper left-hand symbol indicates the presence or absence of the posterior-paralytic phenotype in third instar larvae. The lower right-hand symbol indicates the abundance of axon swellings typically found with each genotype (with the use of anti-synaptotagmin): +, just a few axon swellings per animal (overlaps wild type, i.e., most nerves have no swellings); J, multiple nerves have a few axon swellings; JJ, all nerves have axon swellings but some parts of nerves are free from swellings; JJJ, all nerves have axon swellings, and there are a few small areas of wild-type staining; JJJJ, large swellings predominate the entire width and length of nerves. These designations are qualitative and represent the typical phenotype from each genotype, although variation generated individuals with more or fewer swellings. a, the tail-flipping phenotype was expressed at low penetrance (10–20% compared with ≥50% for the other genotypes).
Similar articles
- Kinesin-1 and Dynein are the primary motors for fast transport of mitochondria in Drosophila motor axons.
Pilling AD, Horiuchi D, Lively CM, Saxton WM. Pilling AD, et al. Mol Biol Cell. 2006 Apr;17(4):2057-68. doi: 10.1091/mbc.e05-06-0526. Epub 2006 Feb 8. Mol Biol Cell. 2006. PMID: 16467387 Free PMC article. - The interaction between cytoplasmic dynein and dynactin is required for fast axonal transport.
Waterman-Storer CM, Karki SB, Kuznetsov SA, Tabb JS, Weiss DG, Langford GM, Holzbaur EL. Waterman-Storer CM, et al. Proc Natl Acad Sci U S A. 1997 Oct 28;94(22):12180-5. doi: 10.1073/pnas.94.22.12180. Proc Natl Acad Sci U S A. 1997. PMID: 9342383 Free PMC article. - Tight functional coupling of kinesin-1A and dynein motors in the bidirectional transport of neurofilaments.
Uchida A, Alami NH, Brown A. Uchida A, et al. Mol Biol Cell. 2009 Dec;20(23):4997-5006. doi: 10.1091/mbc.e09-04-0304. Epub 2009 Oct 7. Mol Biol Cell. 2009. PMID: 19812246 Free PMC article. - Disruption of axonal transport in motor neuron diseases.
Ikenaka K, Katsuno M, Kawai K, Ishigaki S, Tanaka F, Sobue G. Ikenaka K, et al. Int J Mol Sci. 2012;13(1):1225-1238. doi: 10.3390/ijms13011225. Epub 2012 Jan 23. Int J Mol Sci. 2012. PMID: 22312314 Free PMC article. Review. - Cytoplasmic dynein and microtubule transport in the axon: the action connection.
Pfister KK. Pfister KK. Mol Neurobiol. 1999 Oct-Dec;20(2-3):81-91. doi: 10.1007/BF02742435. Mol Neurobiol. 1999. PMID: 10966115 Review.
Cited by
- Normal dynactin complex function during synapse growth in Drosophila requires membrane binding by Arfaptin.
Chang L, Kreko T, Davison H, Cusmano T, Wu Y, Rothenfluh A, Eaton BA. Chang L, et al. Mol Biol Cell. 2013 Jun;24(11):1749-64, S1-5. doi: 10.1091/mbc.E12-09-0697. Epub 2013 Apr 17. Mol Biol Cell. 2013. PMID: 23596322 Free PMC article. - Axonal transport disruption in peripheral nerve disease: From Jack's discoveries as a resident to recent contributions.
Lloyd TE. Lloyd TE. J Peripher Nerv Syst. 2012 Dec;17 Suppl 3(0 3):46-51. doi: 10.1111/j.1529-8027.2012.00431.x. J Peripher Nerv Syst. 2012. PMID: 23279432 Free PMC article. Review. - Dynactin is required for coordinated bidirectional motility, but not for dynein membrane attachment.
Haghnia M, Cavalli V, Shah SB, Schimmelpfeng K, Brusch R, Yang G, Herrera C, Pilling A, Goldstein LS. Haghnia M, et al. Mol Biol Cell. 2007 Jun;18(6):2081-9. doi: 10.1091/mbc.e06-08-0695. Epub 2007 Mar 14. Mol Biol Cell. 2007. PMID: 17360970 Free PMC article. - Selective motor activation in organelle transport along axons.
Cason SE, Holzbaur ELF. Cason SE, et al. Nat Rev Mol Cell Biol. 2022 Nov;23(11):699-714. doi: 10.1038/s41580-022-00491-w. Epub 2022 May 30. Nat Rev Mol Cell Biol. 2022. PMID: 35637414 Review. - Mitochondrial dysfunction in Alzheimer's disease: Guiding the path to targeted therapies.
McGill Percy KC, Liu Z, Qi X. McGill Percy KC, et al. Neurotherapeutics. 2025 Apr;22(3):e00525. doi: 10.1016/j.neurot.2025.e00525. Epub 2025 Jan 17. Neurotherapeutics. 2025. PMID: 39827052 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM044757/GM/NIGMS NIH HHS/United States
- R56 GM044757/GM/NIGMS NIH HHS/United States
- GM46295/GM/NIGMS NIH HHS/United States
- R01 GM046295/GM/NIGMS NIH HHS/United States
- GM44757/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases